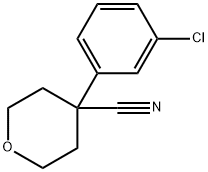
4-(3-chlorophenyl)-tetrahydro-2H-pyran-4-carbonitrile synthesis
- Product Name:4-(3-chlorophenyl)-tetrahydro-2H-pyran-4-carbonitrile
- CAS Number:473706-22-8
- Molecular formula:C12H12ClNO
- Molecular Weight:221.68
Yield:-
Reaction Conditions:
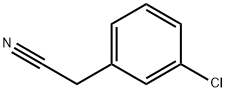
Stage #1: 3-chloro-benzeneacetonitrilewith potassium tert-butylate in N,N-dimethyl-formamide at 0; for 0.166667 h;
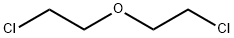
Stage #2: 3-oxa-1,5-dichloropentane in N,N-dimethyl-formamide at 0 - 20; for 20 h;
Steps:
46.A
Step A: Preparation of 4-(3-Chlorophenyl)-tetrahydro-2H-pyran-4-carbonitrile; [0589] Potassium tert-butoxide (8.90 g, 79.3 mmol, 2.20 eq) was added to a stirred solution of 2-(3-chlorophenyl)acetonitrile (4.26 mL, 36.1 mmol) in DMF (50 mL) at 00C under a nitrogen atmosphere. The mixture was stirred for 10 minutes at O0C before l-chloro-2-(2-chloroethoxy)ethane (4.65 mL, 39.7
References:
WO2008/76427,2008,A2 Location in patent:Page/Page column 137-138