
tert-butyl 4-(2-amino-4-bromophenyl)piperazine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(2-amino-4-bromophenyl)piperazine-1-carboxylate
- CAS Number:474329-58-3
- Molecular formula:C15H22BrN3O2
- Molecular Weight:356.26
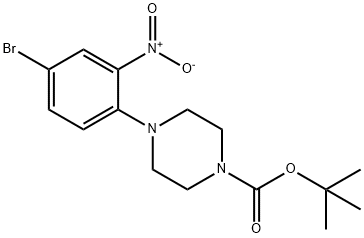
474329-57-2
9 suppliers
inquiry

474329-58-3
6 suppliers
inquiry
Yield:474329-58-3 97%
Reaction Conditions:
with sodium dithionate;sodium carbonate in methanol;water at 70;
Steps:
Synthesis of 2-(4-substitutedpiperazin-1-yl)-5-bromoaniline
General procedure: The nitro compound 1a-b (6.65 mmol) was dissolved in methanol (25 mL) and was added drop wise to the aqueous solution of sodium carbonate (6.5 g, 61.3 mmol) and sodium dithionite (13 g, 74.4 mmol) in 100 ml water at 70 °C. When all of the nitro starting material is consumed, the methanol was evaporated under reduced pressure and the resultant aqueous mixture was extracted with ethyl acetate and washed with water, brine, and dried over Na2SO4, filtered, and concentrated to give the amine product 2a-b as pale yellow foam
References:
Edraki, Najmeh;Firuzi, Omidreza;Foroumadi, Alireza;Miri, Ramin;Madadkar-Sobhani, Armin;Khoshneviszadeh, Mehdi;Shafiee, Abbas [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 8,p. 2396 - 2412]
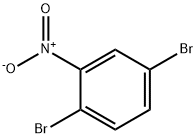
3460-18-2
286 suppliers
$6.00/10g

474329-58-3
6 suppliers
inquiry

57260-71-6
738 suppliers
$5.00/5g

474329-58-3
6 suppliers
inquiry