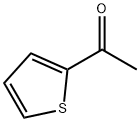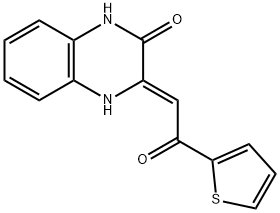
(3Z)-3-[2-oxo-2-(thiophen-2-yl)ethylidene]-1,2,3,4-tetrahydroquinoxalin-2-one synthesis
- Product Name:(3Z)-3-[2-oxo-2-(thiophen-2-yl)ethylidene]-1,2,3,4-tetrahydroquinoxalin-2-one
- CAS Number:476615-56-2
- Molecular formula:C14H10N2O2S
- Molecular Weight:270.31
Yield:476615-56-2 57%
Reaction Conditions:
with methanesulfonic acid in acetonitrile at 20; for 0.25 h;Irradiation;
Steps:
2.2 General procedure for the rapid alkenylation of quinoxalin-2(1H)-ones
General procedure: To a 15 mL tube was added quinoxalin-2(1H)-ones (1) (0.2 mmol), methyl ketones (2) (for acetone (1.0 mL); for other ketones (2.0 equiv.) and acetonitrile (1.0 mL)), CH3SO3H (25 mol%). The above mixture was stirred under the sunlight for 15 minutes. After the completion (as indicated by TLC), the reaction was quenched with saturated NaHCO3. The resulting aqueous phase was then extracted with ethyl acetate and the collected organic layer was washed with brine, dried with MgSO4. The solvent was removed under reduced pressure, and the crude product was further purified by silica gel column chromatography (200-300 mesh silica gel, PE/EA = 3:1) to afford the target product.
References:
Huang, Lin;Xu, Jun;He, Lei;Liang, Chenfeng;Ouyang, Yani;Yu, Yongping;Li, Wanmei;Zhang, Pengfei [Chinese Chemical Letters,2021,vol. 32,# 11,p. 3627 - 3631] Location in patent:supporting information
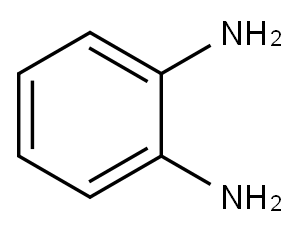
95-54-5
494 suppliers
$9.00/1g
![(3Z)-3-[2-oxo-2-(thiophen-2-yl)ethylidene]-1,2,3,4-tetrahydroquinoxalin-2-one](/CAS/20180529/GIF/476615-56-2.gif)
476615-56-2
1 suppliers
inquiry