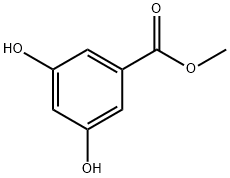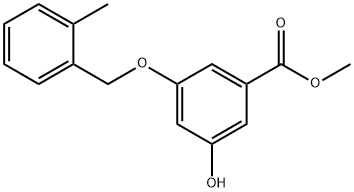
Benzoic acid, 3-hydroxy-5-[(2-methylphenyl)methoxy]-, methyl ester synthesis
- Product Name:Benzoic acid, 3-hydroxy-5-[(2-methylphenyl)methoxy]-, methyl ester
- CAS Number:480464-58-2
- Molecular formula:C16H16O4
- Molecular Weight:272.3
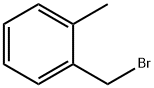
89-92-9
141 suppliers
$27.00/5g
![Benzoic acid, 3-hydroxy-5-[(2-methylphenyl)methoxy]-, methyl ester](/CAS/20210305/GIF/480464-58-2.gif)
480464-58-2
0 suppliers
inquiry
Yield:480464-58-2 27%
Reaction Conditions:
Stage #1: 1-carbomethoxy-3,5-dihydroxybenzenewith sodium hydride in N,N-dimethyl-formamide at 0 - 15; for 0.333333 h;
Stage #2: 2-methylbenzyl bromide in N,N-dimethyl-formamide at 0 - 20; for 0.5 h;
Steps:
H.A
To a solution of methyl 3,5-dihydroxybenzoate (50 g, 0.30 mol) in DMF (500 ml) at 0 0C was added sodium hydride (10.8 g, 0.27 mol) portion wise. The reaction was allowed to warm to 15 0C, and stirred for 20 minutes. The mixture was re-cooled to 0 0C and a solution of 2- methylbenzyl bromide (36 ml, 0.27 mol) in DMF (50 ml) was added over 30 minutes. The reaction was warmed to ambient temperature and concentrated in vacuo. The residual oil was partitioned between ethyl acetate (500 ml) and water (250 ml), and the ethyl acetate layer was separated, washed with water and evaporated. The residue was purified by column chromatograph on silica gel (gradient, 0-100% ethyl acetate in isohexane) to give the desired compound (21.9 g, 27%). 1H NMR (300 MHz, CDCI3) δ: 7.15-7.42 (m, 6 H), 6.69 (t, 1 H), 5.61 (s, 1 H), 5.02 (s, 1 H), 3.90 (s, 3 H), 2.39 (s, 3 H).
References:
WO2007/125103,2007,A2 Location in patent:Page/Page column 51