2H-1-Benzopyran-3-carboxylic acid, 7-chloro-, ethyl ester synthesis
- Product Name:2H-1-Benzopyran-3-carboxylic acid, 7-chloro-, ethyl ester
- CAS Number:482374-63-0
- Molecular formula:C12H11ClO3
- Molecular Weight:238.67

20345-61-3
23 suppliers
inquiry

482374-63-0
0 suppliers
inquiry
Yield:-
Reaction Conditions:
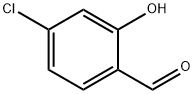
Stage #1: 4-chlorosalicylaldehydewith sodium hydride in tetrahydrofuran at 20; for 2 h;
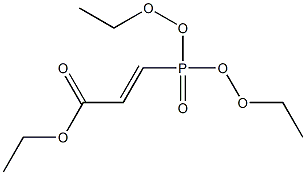
Stage #2: ethyl 2-diethoxyphosphinoylacrylate in tetrahydrofuran at 20;Heating / reflux;
Steps:
305
4-Chloro-2-hydroxybenzaldehyde (Acta. Chem. Scand., vol. 53, p. 258 (1999)) (510 mg) was dissolved in tetrahydrofuran (40 mL), and sodium hydride (60% in oil, 157 mg) was added thereto, followed by stirring at room temperature for 2 hours. To the reaction mixture was added a solution of 2-diethylphosphonoacrylic acid ethyl ester (J. Org. Chem., vol. 43, p. 1256 (1978)) (769 mg) in tetrahydrofuran (10 mL), and the thus-obtained mixture was stirred at room temperature for 2 hours, followed by heating under reflux overnight. After the reaction mixture was cooled to room temperature, the mixture was partitioned between water and diethyl ether. The organic layer was dried over sodium sulfate anhydrate, and the solvent was distilled away under reduced pressure. The residue was purified by silica gel column chromatography (hexane : ethyl acetate = 10:1), to thereby give the title compound (247 mg).1H-NMR(DMSO-d6) δ:1.33(3H, t, J=7.1Hz), 4.27(2H, q, J=7.1Hz), 4.99(2H, d, J=1.2Hz), 6.85(1H, d, J=1.2Hz), 6.89(1H, dd, J=8.1, 2.0Hz), 7.04(1H, d, J=8.1Hz), 7.38(1H, d, J=1.0Hz). MS(EI)m/z:238(M+).
References:
EP1577301,2005,A1 Location in patent:Page/Page column 222-223