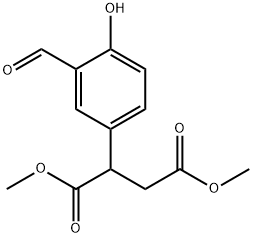
Butanedioic acid, 2-(3-forMyl-4-hydroxyphenyl)-, 1,4-diMethyl ester synthesis
- Product Name:Butanedioic acid, 2-(3-forMyl-4-hydroxyphenyl)-, 1,4-diMethyl ester
- CAS Number:488713-20-8
- Molecular formula:C13H14O6
- Molecular Weight:266.25
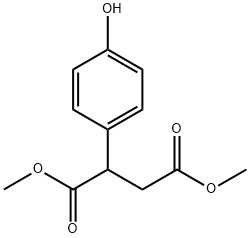
136705-25-4
10 suppliers
inquiry

488713-20-8
5 suppliers
inquiry
Yield:488713-20-8 89%
Reaction Conditions:
with hydrogenchloride;triethanolamine;magnesium chloride;paraformaldehyde in acetonitrile;
Steps:
d Step (d)
Step (d) A mixture of 2-(4-hydroxy-phenyl)-succinic acid dimethyl ester (11.90 g, 50.0 mmol) and dry acetonitrile (250 mL) was treated with anhydrous magnesium chloride (7.14 g, 75.0 mmol), TEA (26.13 mL, 0.1875 mol) and paraformaldehyde (10.51 g, 0.35 mol). The reaction mixture was refluxed for approximately 1 hour, cooled to ambient temperature and mixed with 1N HCl/ether. The organic layer was isolated and the aqueous layer was extracted with ether (*2). The combined organic extracts were dried (MgSO4) and concentrated under reduced pressure. A mixture of the residue and dry acetonitrile (250 mL) was treated with anhydrous magnesium chloride (7.14 g, 75.0 mmol), TEA (26.13 mL, 0.1875 mol) and paraformaldehyde (10.51 g, 0.35 mol). The reaction mixture was refluxed for approximately 1 hour, cooled to ambient temperature and mixed with 1N HCl/ether. The organic layer was isolated and the aqueous layer was extracted with ether (*2). The combined organic extracts were dried (MgSO4) and concentrated under reduced pressure to yield 2-(3-formyl-4-hydroxy-phenyl)-succinic acid dimethyl ester (89% yield).
References:
US2003/114457,2003,A1
23053-35-2
0 suppliers
inquiry

488713-20-8
5 suppliers
inquiry

22248-26-6
10 suppliers
inquiry

488713-20-8
5 suppliers
inquiry
