
3-(CHLOROMETHYL)-5-(3-FLUOROPHENYL)-1,2,4-OXADIAZOLE synthesis
- Product Name:3-(CHLOROMETHYL)-5-(3-FLUOROPHENYL)-1,2,4-OXADIAZOLE
- CAS Number:491842-63-8
- Molecular formula:C9H6ClFN2O
- Molecular Weight:212.61
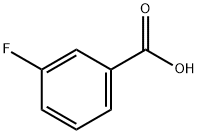
455-38-9
380 suppliers
$11.92/25g
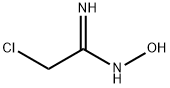
3272-96-6
33 suppliers
$85.00/250mg

491842-63-8
22 suppliers
inquiry
Yield:491842-63-8 35%
Reaction Conditions:
Stage #1: 3-fluorobenzoic acid;2-chloro-N'-hydroxyacetimidamidewith benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in DMF (N,N-dimethyl-formamide) at 20;
Stage #2: in DMF (N,N-dimethyl-formamide) at 135; for 3.5 h;
Steps:
16 Example 16; 3-CHLOROMETHYL-5- (3-FLUORO-PHENYL)- [1,2,4]oxadiazole
DMF (10 ml) was added to a mixture of 3-fluorobenzoic acid (710 mg, 5.07 mmol), [1- (3-] dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) (972 mg, 5.07 mmol), 1-hydroxybenzotriazole hydrate (HOBt) [(685] mg, 5.07 mmol) and 2-chloro-N-hydroxy- acetamidine (500 mg, 4.61 mmol) at room temperature and then stirred overnight. The reaction mixture was diluted with ethyl acetate, washed with water (3 times) and brine, dried over anhydrous sodium sulfate, filtered and concentrated. DMF (14 ml) was added to the residue and the resulting solution was heated at [135] for 3.5 h to effect cyclization to oxadiazole. After cooling the reaction mixture was washed with water (3 times) and brine, dried over anhydrous sodium sulfate, filtered and concentrated. [3-CHLOROMETHYL-5- (3-] [FLUORO-PHENYL)- [1,] 2,4] oxadiazole [(383] mg, 35% yield over 2 steps, yellow oil) was obtained by flash chromatography on silica gel, using 5% ethyl acetate in hexanes NMR [(CDC13),] [8] (ppm): 7.96 (d, 1H), 7.86 (m, 1H), 7.54 (m, [1H),] 7.33 (m, 1H), 4. [68] (s, 2H).
References:
WO2004/14881,2004,A2 Location in patent:Page 105
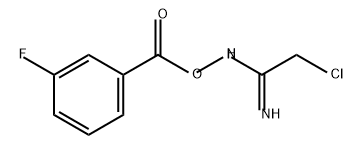
844498-87-9
0 suppliers
inquiry

491842-63-8
22 suppliers
inquiry

3272-96-6
33 suppliers
$85.00/250mg
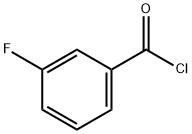
1711-07-5
325 suppliers
$6.00/5g

491842-63-8
22 suppliers
inquiry

1711-07-5
325 suppliers
$6.00/5g

491842-63-8
22 suppliers
inquiry

844498-87-9
0 suppliers
inquiry

491842-63-8
22 suppliers
inquiry