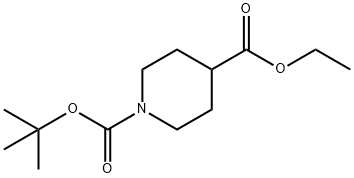
1,4-Piperidinedicarboxylic acid, 4-(cyanomethyl)-, 1-(1,1-dimethylethyl) 4-ethyl ester synthesis
- Product Name:1,4-Piperidinedicarboxylic acid, 4-(cyanomethyl)-, 1-(1,1-dimethylethyl) 4-ethyl ester
- CAS Number:495414-81-8
- Molecular formula:C15H24N2O4
- Molecular Weight:296.36
Yield:495414-81-8 42.6%
Reaction Conditions:
Stage #1: ethyl 1-tert-butyloxycarbonyl-4-piperidinecarboxylatewith lithium dipropan-2-ylazanide in tetrahydrofuran at -78; for 1 h;
Stage #2: cyanomethyl bromide in tetrahydrofuran at 20; for 12 h;
Steps:
1.1.3. Synthetic procedure for 1-tert-butyl 4-ethyl 4-(cyanomethyl)piperidine-1,4-dicarboxylate c
Compound b (5.20 g, 20.2 mmol) was dissolved in tetrahydrofuran (60 mL) and cooled to -78°C, and lithium diisopropylamide (2 M, 11.1 mL, 22.2 mmol) was added to the solution. After 1.0 h of stirring, bromoacetonitrile (1.7 mL, 24.2 mmol) was added and the resulting solution was allowed to warm to room temperature over 12 h. The reaction was quenched by the addition of water (10 mL) and extracted with ethyl acetate (3×60 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated in vacuum. The residue was purified by silica gel column chromatography eluting with ethyl acetate/petroleum ether (1/10-1:5, v/v) to obtain the desired product c (2.56 g) as yellow oil liquid, yield: 42.6%. 1H NMR (600 MHz, CDCl3) δ 4.26 (q, J = 7.0 Hz, 2H, CH3CH2O), 3.82 (s, 2H, piperidine-CH2), 3.08 (s, 2H, piperidine-CH2), 2.61 (s, 2H, CH2), 2.16 (d, J = 13.5 Hz, 2H, piperidine-CH2), 1.56 (dd, J=16.9, 7.2 Hz, 2H, piperidine-CH2), 1.46 (s, 9H, C(CH3)3), 1.31 (q, J = 7.0 Hz, 3H, CH3CH2O).
References:
Li, Bing;Wang, Kaiyuan;Zhang, Rui;Li, Baihui;Shen, Yangli;Ji, Qinggang [European Journal of Medicinal Chemistry,2019,vol. 182,art. no. 111669] Location in patent:supporting information
498-94-2
492 suppliers
$5.00/10g

495414-81-8
30 suppliers
inquiry

24424-99-5
820 suppliers
$13.50/25G

495414-81-8
30 suppliers
inquiry