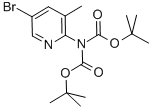
DI-TERT-BUTYL [5-BROMO-3-METHYLPYRIDIN-2-YL]IMIDODICARBONATE synthesis
- Product Name:DI-TERT-BUTYL [5-BROMO-3-METHYLPYRIDIN-2-YL]IMIDODICARBONATE
- CAS Number:497159-91-8
- Molecular formula:C16H23BrN2O4
- Molecular Weight:387.27

24424-99-5
821 suppliers
$13.50/25G
3430-21-5
398 suppliers
$5.00/1g
![DI-TERT-BUTYL [5-BROMO-3-METHYLPYRIDIN-2-YL]IMIDODICARBONATE](/CAS/GIF/497159-91-8.gif)
497159-91-8
18 suppliers
$150.00/1g
Yield:497159-91-8 77%
Reaction Conditions:
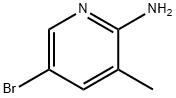
Stage #1: 2-amino-5-bromo-3-methyl pyridinewith sodium bis-(trimethyl-silyl)amide in tetrahydrofuran; for 0.25 h;
Stage #2: di-tert-butyl dicarbonatewith N,N-dimethyl-4-aminopyridine at 20; for 16 h;
Steps:
1.1 [Step-1] Preparation of tert-butyl (5-bromo-3-methylpyridin-2-yl)(tert-butoxycarbonyl)carbamate
After 5-bromo-3-methylpyridin-2-amine (1.0 equiv.) was dissolved in THF (0.53M), a 1M solution of sodium bis(trimethylsilyl)amide (2.0 equiv.) was added, followed by stirring for 15 minutes. Then, di-tert-butyldicarbonate (6.0 equivalents) and 4-(dimethylamino)pyridine (0.2 equivalents) were added, followed by stirring at room temperature for 16 hours. After confirming the product using LC-MS, the reaction mixture was concentrated and diluted with DCM, and the organic layer was washed with distilled water. The obtained organic layer was dried over MgSO4 and then concentrated under reduced pressure. The concentrated material was purified by MPLC (Hx:EA) to obtain the target compound (yield: 77 %, MS (ESI): m/z 388 [M+1]+).
References:
KR2022/99503,2022,A Location in patent:Paragraph 0155-0159