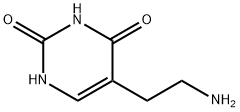
5-(2-aminoethyl)pyrimidine-2,4(1H,3H)-dione synthesis
- Product Name:5-(2-aminoethyl)pyrimidine-2,4(1H,3H)-dione
- CAS Number:221170-25-8
- Molecular formula:C6H9N3O2
- Molecular Weight:155.15
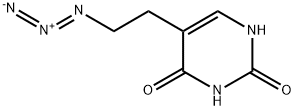
221170-22-5
7 suppliers
inquiry

221170-25-8
10 suppliers
$58.00/100mg
Yield:221170-25-8 85%
Reaction Conditions:
Stage #1: 5-(2-azidoethyl)uracilwith triphenylphosphine in pyridine at 20 - 60;
Stage #2: with ammonia in pyridine;water;
Steps:
1.1 1. Procedure
14.2 g (78.0 mmol) of 30 were suspended in 175 ml of pyridine in a 250 ml three-necked flask equipped with an internal thermometer and reflux condenser and treated with 61.4 g (234 mmol) of triphenylphosphine2). The mixture was heated at 60° C. for five hours and stirred overnight at room temp. (TLC checking, CHCl3/MeOH 5:1). 40 ml of a 25% strength ammonia solution were added to the solution, which then clarified. The solvents were removed in vacuo in a rotary evaporator. The residue was stirred at room temperature for 30 min in 200 ml of CH2Cl2/MeOH 1:1, and the precipitate was filtered off and washed with CH2Cl2. After drying in vacuo in a desiccator over phosphorus pentoxide, 10.0 g (85%) of 31 of m.p. 214-220° C. were obtained. [00093] 2. Analytical Data5-(2-Aminoethyl)uracil (31) [00094] M.P.: 214-220° C. and with evolution of gas, presintering. DC: CHCl3/MeOH/HOAc/H2O 85:20:10:2, Rf 0.07. UV (MeOH): λmax 263.0 (6400). IR (KBr): 3430m, 3109s, 1628s, 1474m, 1394s, 1270s, 1176w, 1103m, 1021m, 966m, 908m, 838m. 1H-NMR (300 MHz, d6-DMSO): 2.21 (t, 2H, J(CH2CH2N, CH2CH2N)=6.8, CH2CH2N); 2.59 (t, 2H, J(CH2CH2N, CH2CH2N) =6.8, CH2CH2N); 5.90 (v. br. s, 4H, H-N(1), H-N(3), NH2); 7.19 (s, H-C(6)). MS (ESI-): 153.9 [M-H].
References:
US6699978,2004,B1 Location in patent:Page column 10-14
![Urea, N-[(dihydro-2-oxo-3(2H)-furanylidene)methyl]-](/CAS/20210305/GIF/89532-90-1.gif)
89532-90-1
0 suppliers
inquiry

221170-25-8
10 suppliers
$58.00/100mg
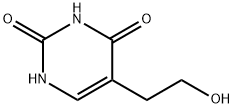
23956-12-9
47 suppliers
$26.00/250mg

221170-25-8
10 suppliers
$58.00/100mg
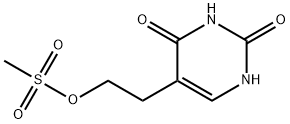
37107-76-9
3 suppliers
inquiry

221170-25-8
10 suppliers
$58.00/100mg