
5-(2-FURYL)-4-METHYL-4H-1,2,4-TRIAZOLE-3-THIOL synthesis
- Product Name:5-(2-FURYL)-4-METHYL-4H-1,2,4-TRIAZOLE-3-THIOL
- CAS Number:27106-14-5
- Molecular formula:C7H7N3OS
- Molecular Weight:181.21

41735-61-9
7 suppliers
inquiry

27106-14-5
20 suppliers
$126.00/500mg
Yield:-
Reaction Conditions:
Stage #1: 1-(2-furoyl)-4-methyl-3-thiosemicarbazidewith sodium hydrogencarbonate in water at 100;
Stage #2: with hydrogenchloride in water at 0; pH=6;
Steps:
31 Example 31; 5-FURAN-2-YL-4-METHYL-4H-[1, 2,4]triazole-3-thiol
2-Furoyl chloride (0.76 ml, 7.66 mmol) was added in a dropwise manner to a solution of 4- methyl-3-thiosemicarbazide (732 mg, 6.96 mmol) and pyridine (7 ml) and the resulting solution was stirred at room temperature for 4 h. The reaction mixture was diluted with ethyl acetate (100 ml), successively washed with water [(3X100] ml) and brine (100 ml). The organic phase was dried (sodium sulfate), filtered and concentrated in-vacuo. The residue was suspended in sodium bicarbonate (70 ml, 69.6 mmol, 1 M water) and left stirring at [100°C] overnight. The reaction mixture was cooled to [0°C,] then brought to pH about 6 using hydrochloric acid (70 ml, 1 N water). The title compound [(298] mg) was collected by filtration as a white [SOLID. 1H] NMR [(CDC13),] [8] (ppm): 11.4 (bs, 1H), 7.63 (d, 1H), 7.02 (d, 1H), 6.60 (dd, 1H), 3. 83 (s, 3H).
References:
WO2004/14881,2004,A2 Location in patent:Page 110
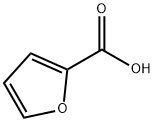
88-14-2
483 suppliers
$5.00/10g
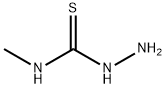
6610-29-3
203 suppliers
$19.00/1g

27106-14-5
20 suppliers
$126.00/500mg
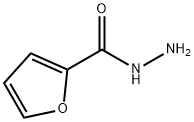
3326-71-4
219 suppliers
$15.00/5mg

27106-14-5
20 suppliers
$126.00/500mg