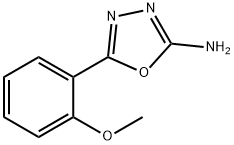
5-(2-METHOXYPHENYL)-1,3,4-OXADIAZOL-2-AMINE synthesis
- Product Name:5-(2-METHOXYPHENYL)-1,3,4-OXADIAZOL-2-AMINE
- CAS Number:5711-59-1
- Molecular formula:C9H9N3O2
- Molecular Weight:191.19
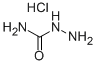
563-41-7
550 suppliers
$5.00/25g
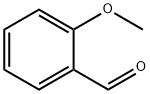
135-02-4
412 suppliers
$10.00/10g

5711-59-1
25 suppliers
$175.00/250mg
Yield:5711-59-1 70%
Reaction Conditions:
Stage #1: hydrazinecarboxamide monohydrochloride;ortho-anisaldehydewith anhydrous Sodium acetate in tetrahydrofuran;lithium hydroxide monohydrate; for 1.5 h;Cooling with ice;
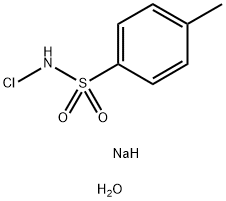
Stage #2: with chloramine-T;potassium carbonate in tetrahydrofuran;lithium hydroxide monohydrate at 20; for 3 h;
Steps:
C14 Example C14
Example C14
Production of 5-(2-methoxyphenyl)-1,3,4-oxadiazol-2-amine
A solution of semicarbazide hydrochloride (1.22 g, 10.9 mmol) and sodium acetate (0.897 g, 10.9 mmol) in water (10 mL) was cooled in ice, then 2-methoxybenzaldehyde (1.42 g, 10.4 mmol) and tetrahydrofuran (THF) (3.0 mL) were added thereto, and the mixture was stirred. 1.5 hours later, tetrahydrofuran (THF) (27 mL), potassium carbonate (3.60 g, 26.0 mmol), and chloramine T trihydrate (4.11 g, 14.6 mmol) were added to the reaction solution, and the mixture was stirred at room temperature. 3 hours later, the reaction solution was washed with a 2:1 mixed solution of a 20% sodium bisulfite aqueous solution and 30% aqueous sodium chloride solution. 4 mol/L hydrochloric acid in ethyl acetate (7.8 mL, 31.2 mol) and toluene were added to the obtained organic layer, followed by extraction with a 4:1 mixed solution of 2 mol/L hydrochloric acid and 30% aqueous sodium chloride solution.
The obtained aqueous layer was adjusted to pH 12 or higher by the addition of a 10 mol/L sodium hydroxide aqueous solution.
After overnight stirring, the resulting solid was collected by filtration, washed with water, and then dried under reduced pressure to obtain the title compound (1.39 g, yield: 70%) as a white solid.
1H-NMR (400 MHz, DMSO-D6) δ: 7.64 (1H, dd, J=7.3, 1.8 Hz), 7.53-7.48 (1H, m), 7.20 (1H, d, J=8.5 Hz), 7.12 (2H, brs), 7.09-7.04 (1H, m), 3.85 (3H, s)
ESI-MS (m/z): 192 (M+H)+
References:
US2022/169640,2022,A1 Location in patent:Paragraph 0299-0301

563-41-7
550 suppliers
$5.00/25g
7080-50-4
284 suppliers
$7.00/25g

135-02-4
412 suppliers
$10.00/10g

5711-59-1
25 suppliers
$175.00/250mg
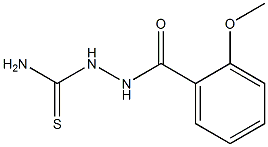
7653-42-1
2 suppliers
inquiry

5711-59-1
25 suppliers
$175.00/250mg

7466-54-8
98 suppliers
$9.00/5g

5711-59-1
25 suppliers
$175.00/250mg

135-02-4
412 suppliers
$10.00/10g

5711-59-1
25 suppliers
$175.00/250mg