
5-(2-NITRO-PHENOXYMETHYL)-FURAN-2-CARBALDEHYDE synthesis
- Product Name:5-(2-NITRO-PHENOXYMETHYL)-FURAN-2-CARBALDEHYDE
- CAS Number:438221-76-2
- Molecular formula:C12H9NO5
- Molecular Weight:247.2
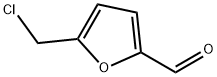
1623-88-7
78 suppliers
$60.00/25mg
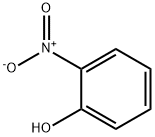
88-75-5
9 suppliers
$15.00/5mg

438221-76-2
17 suppliers
$240.00/0.500g
Yield:438221-76-2 77%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide;acetonitrile;Reflux;
Steps:
5-Aryloxymethylfurfurals 8a,b (general procedure).
General procedure: A mixture of 5-chloromethylfurfural36 (0.010 mol), the corresponding phenol (0.011 mol), and potassium carbonate (2.00 g, 0.0145 mol)in a mixture of acetonitrile-DMF (20 mL, 9 : 1, v/v) was refluxed for 5-6 h with stirring (TLC monitoring). After evaporation of the solvents, the residue was treated with water, a precipitate formed was filtered off, washed with 30% aqueous methanol, and dried in air. Yields and physicochemical characteristics of aldehydes 8a,b are given in Table 4.
References:
Khachatryan;Razinov;Kolotaev;Belus?;Matevosyan [Russian Chemical Bulletin,2015,vol. 64,# 2,p. 395 - 404][Izv. Akad. Nauk, Ser. Khim.,2015,# 2,p. 395 - 404,10]