
5-(3-METHOXYPHENYL)-5-OXOVALERIC ACID synthesis
- Product Name:5-(3-METHOXYPHENYL)-5-OXOVALERIC ACID
- CAS Number:845781-34-2
- Molecular formula:C12H14O4
- Molecular Weight:222.24

108-55-4
449 suppliers
$6.00/25g
2398-37-0
583 suppliers
$8.00/10g

845781-34-2
18 suppliers
$303.00/1g
Yield:845781-34-2 82%
Reaction Conditions:
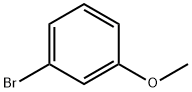
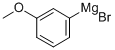
Stage #1: 3-methoxyphenyl bromidewith iodine;magnesium in tetrahydrofuran;Heating;Large scale;
Stage #2: glutaric anhydride,with iodine;magnesium in tetrahydrofuran at 0 - 20;Large scale;
Steps:
1.1 (1) Synthesis of 5-(3-methoxyphenyl)-5-oxopentanoic acid
Magnesium turnings (15.9 kg, 1.1 eq), iodine (0.05 kg) and m-bromoanisole (11.0 kg) were added to a solution of tetrahydrofuran (290 kg) in that order.The reaction was initiated with heating and then a solution of m-bromoanisole (96.0 kg) in tetrahydrofuran (480 kg) was added dropwise.After the addition was completed, the reaction system was reacted for 2-3 h to obtain a solution (a).The solution (a) was then added dropwise to a solution of glutaric anhydride (137.0 kg, 2.0 eq) in tetrahydrofuran (240 kg) at 0-5°C. After the addition was complete, the reaction was allowed to proceed to room temperature for 6-8 h.After the reaction is completed, the mixture is acidified with 1N hydrochloric acid at 0-5° C. to a pH of 1-2, tetrahydrofuran is concentrated under reduced pressure, and the product is extracted with methyl tert-butyl ether (110 kg,).Then use 10% sodium hydroxide solution to wash the product into the aqueous phase, adjust the pH of the aqueous phase to 1-2 with 3N hydrochloric acid, stir and crystallization, and filter to obtain a solid.The filter cake was rinsed with a small amount of water and dried to give 5-(3-methoxyphenyl)-5-oxopentanoic acid (104.0 kg). Yield: 82%.
References:
CN107501061,2017,A Location in patent:Paragraph 0048; 0049; 0050

108-55-4
449 suppliers
$6.00/25g
36282-40-3
101 suppliers
$112.00/100ml

845781-34-2
18 suppliers
$303.00/1g