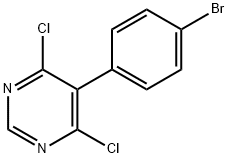
5-(4-Bromophenyl)-4,6-dichloropyrimidine synthesis
- Product Name:5-(4-Bromophenyl)-4,6-dichloropyrimidine
- CAS Number:146533-41-7
- Molecular formula:C10H5BrCl2N2
- Molecular Weight:303.97
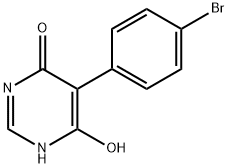
706811-25-8
98 suppliers
$12.00/1g

146533-41-7
308 suppliers
$16.00/1g
Yield:146533-41-7 86%
Reaction Conditions:
with trichlorophosphate in water;N,N-dimethyl-aniline;toluene at 30 - 100; for 5 h;Temperature;
Steps:
3.4 Step four:
200 g of the intermediate 3 obtained in the third step was taken in a 3 L three-necked flask.Add 300g of toluene and 180g of N,N-dimethylaniline, mechanically stirred,230 g of phosphorus oxychloride was added dropwise at 30 ° C, and the temperature was raised to 55 ° C after the addition.After the solid is completely dissolved, the temperature is raised to 100 ° C, the reaction is carried out for 4 h, and then cooled to 25 ° C for use.450 g of water was mixed with 500 g of toluene, and cooled to 25 ° C with stirring.The above-mentioned alternate reaction solution was added, and the process control temperature was 30 ° C.Stir at 30 ° C for 1 h, then stand for stratification, and extract the aqueous phase with toluene several times.The toluene extract and the organic phase are combined, and the combined organic phases are decomposed under reduced pressure.Further, ethanol was added, and the mixture was stirred at 15 ° C for 1.5 hours, suction filtered, and dried.The product 5-(4-bromophenyl)-4,6-dichloropyrimidine 195.6 g, the yield was 86.0%,The liquid chromatographic test results of the obtained product are shown in Figure 2.HPLC purity is 99.93%,The test results came to the Waters 2489-1525 high performance liquid chromatograph produced by Waters.
References:
Zhejiang Pioneer Technology Co., Ltd.;Gao Feifei;Gao Junlong;Zhu Minliang;Lu Wei;Liu Yi CN108997223, 2018, A Location in patent:Paragraph 0056; 0063; 0065; 0070; 0077; 0084; 0091
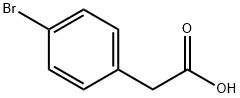
1878-68-8
484 suppliers
$6.00/10g

146533-41-7
308 suppliers
$16.00/1g

146533-40-6
2 suppliers
inquiry

146533-41-7
308 suppliers
$16.00/1g
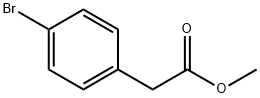
41841-16-1
210 suppliers
$5.00/5g

146533-41-7
308 suppliers
$16.00/1g

149506-35-4
93 suppliers
$43.00/250mg

146533-41-7
308 suppliers
$16.00/1g