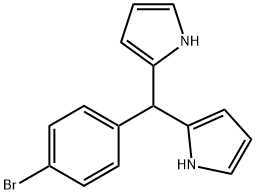
5-(4-Bromophenyl)dipyrromethane synthesis
- Product Name:5-(4-Bromophenyl)dipyrromethane
- CAS Number:159152-11-1
- Molecular formula:C15H13BrN2
- Molecular Weight:301.18
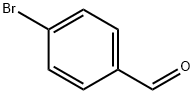
1122-91-4
691 suppliers
$9.00/10g

159152-11-1
16 suppliers
$317.10/1G
Yield:159152-11-1 92%
Reaction Conditions:
Stage #1: 4-bromo-benzaldehydewith indium(III) chloride;pyrrole for 1.5 h;
Stage #2: with sodium hydroxide for 0.75 h;
Steps:
Following the standard procedure,23 4-bromobenzaldehyde (5.55 g, 30.0 mmol) was dissolved in dry pyrrole (208 mL, 3.00 mol), and the solution was flushed with argon for 10 min. InCl3 (666 mg, 3.00 mmol) was added, and the reaction was allowed to proceed for 90 min. The reaction was quenched by addition of powdered NaOH (3.60 g, 90.0 mmol). The mixture was stirred for 45 min. The mixture was filtered. The filtrate was concentrated at reduced pressure. Chromatography [silica, ethyl acetate/CH2Cl2/hexanes (1:2:7)] afforded a pale yellow solid (8.29 g, 92%): mp 120- 122 0C; IR (film, vmax cm"1) 1487; 1H NMR δ 5.43 (s, IH), 5.88-5.89 (m, 2H), 6.15- 6.16 (m, 2H), 6.70-6.71 (m, 2H), 7.06-7.08 (m, 2H) 7.42-7.44 (m, 2H), 7.88-7.93 (br, 2H); 13C NMR δ 43.66, 107.65, 108.80, 112.61, 117.73, 121.08, 130.36, 131.92, 141.40; EI-MS 145, 234/236, 300/302; FAB-MS obsd 300.0257, calcd 300.0262 (C15H13BrN2); Anal. Calcd C, 59.82; H, 4.53; N, 9.30. Found C, 59.99; H, 4.39; N, 9.19.
References:
WO2007/47925,2007,A2 Location in patent:Page/Page column 73; 94
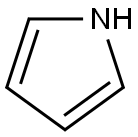
109-97-7
348 suppliers
$9.00/5G

1122-91-4
691 suppliers
$9.00/10g

159152-11-1
16 suppliers
$317.10/1G