
[5-(4-CHLOROPHENYL)PYRIDIN-3-YL]METHANOL synthesis
- Product Name:[5-(4-CHLOROPHENYL)PYRIDIN-3-YL]METHANOL
- CAS Number:887974-02-9
- Molecular formula:C12H10ClNO
- Molecular Weight:219.67
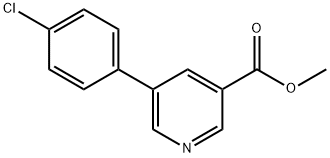
893734-71-9
10 suppliers
inquiry
![[5-(4-CHLOROPHENYL)PYRIDIN-3-YL]METHANOL](/StructureFile/ChemBookStructure8/GIF/CB5103186.gif)
887974-02-9
11 suppliers
inquiry
Yield:887974-02-9 67%
Reaction Conditions:
with sodium tetrahydroborate in ethanol at 20 - 50; for 48 h;
Steps:
38.ii
ii) [5-(4-Chloro-phenyl)-pyridin-3-yl]-methanol A solution of 900 mg (3.49 mmol) 5-(4-Chloro-phenyl)-nicotinic acid methyl ester in 30.0 ml Ethanol was treated at room temperature (r. t.) with 264 mg (69.9 mmol) of sodium borohydride. The mixture was stirred for two days at 50° C. Then it was poured on ice and extracted tree times with ethyl acetate. The combined organic phases were washed with water, dried (sodium sulphate) and evaporated. Chromatography on silica (eluent: ethyl acetate/methanol 6:1) gave 522 mg (67%) of [5-(4-Chloro-phenyl)-pyridin-3-yl]-methanol. MS: 219.9 (ESI+) 1H-NMR(400 Hz, [D6]DMSO): δ=4.61(d, 2H, CH2), 5.37(t, 1H, -OH), 7.56(d, 2H, Ar-Cl), 7.75(d, 2H, Ar-Cl), 7.99(s, 1H, pyridine), 8.54(s, 1H, pyridine), 8.78(s, 1H, pyridine).
References:
US2007/32530,2007,A1 Location in patent:Page/Page column 26