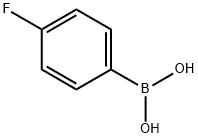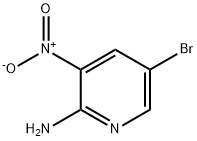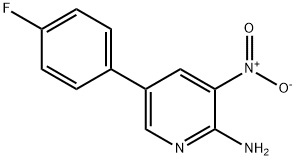
5-(4-Fluorophenyl)-3-nitro-2-pyridinylamine synthesis
- Product Name:5-(4-Fluorophenyl)-3-nitro-2-pyridinylamine
- CAS Number:640271-51-8
- Molecular formula:C11H8FN3O2
- Molecular Weight:233.2
Yield:640271-51-8 76%
Reaction Conditions:
with sodium carbonate;tetrakis(triphenylphosphine) palladium(0) in 1,2-dimethoxyethane;water at 80; for 9 h;
Steps:
26.1 Example 26; Preparation of [TRANS-6- (4-FLUOROPHENYL)-2- [3'-] oxospiro [cyclohexane-1, [1' (3'H)-ISOBENZOFURAN]-4-] [YL] IMIDAZO] [4, [5-B] PYRIDINE]; (1) Preparation of [2-AMINO-5- (4-FLUOROPHENYL)-3-] nitropyridine
To a suspension of 2-amino-5-bromo-3-nitropyridine [(2. 2] [G,] 10.1 mmol) in dimethoxyethane (20 mL) were added [4-FLUOROPHENYLBORONIC] acid (4.83 [G,] 13.1 mmol), tetrakis (triphenylphosphine) palladium (580 mg, 0.50 mmol) and 2N aqueous sodium carbonate solution (10 mL). The mixture was stirred at [80°C] for 9 hours under a nitrogen atmosphere. After the reaction mixture was cooled to room temperature, it was diluted with ethyl acetate to give a precipitate, which was collected by filtration, washed with ethyl acetate and then dried to give the title compound (1.76 g, 76 [%).]
References:
WO2004/2986,2004,A2 Location in patent:Page 116-117