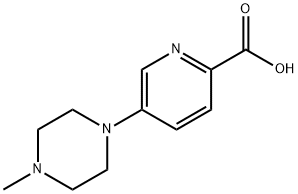
5-(4-methylpiperazin-1-yl)picolinic acid synthesis
- Product Name:5-(4-methylpiperazin-1-yl)picolinic acid
- CAS Number:892501-96-1
- Molecular formula:C11H15N3O2
- Molecular Weight:221.26
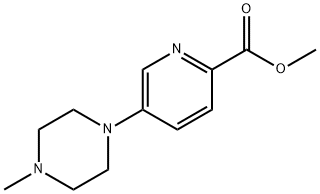
1035270-92-8
4 suppliers
inquiry

892501-96-1
18 suppliers
$110.00/100mg
Yield:892501-96-1 74%
Reaction Conditions:
with lithium hydroxide in methanol;water at 23; for 16 h;
Steps:
6.2 Step 2. 5-(4-methylpiperazin-1-yl)pyridine-2-carboxylic acid
A 50-mL round-bottom flask fitted with a magnetic stir bar was charged with methyl 5-(4-methylpiperazin-1-yl)pyridine-2-carboxylate (Step 1, 130 mg, 0.55 mmol), methanol (10 mL), lithium hydroxide (40 mg, 1.67 mmol), and water (2 mL).
The solution was stirred for 16 h at 23° C. and concentrated under reduced pressure.
The residue was diluted with water (10 mL) and the pH was adjusted to 6 with hydrochloric acid (3N).
The solids were collected by filtration and dried in an oven to afford 5-(4-methylpiperazin-1-yl)pyridine-2-carboxylic acid (Intermediate 2-16, 90 mg, 74%). LCMS (ESI) m/z 222 [M+H].
References:
US2016/229872,2016,A1 Location in patent:Paragraph 0546; 0548