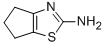
5,6-DIHYDRO-4H-CYCLOPENTATHIAZOL-2-YLAMINE synthesis
- Product Name:5,6-DIHYDRO-4H-CYCLOPENTATHIAZOL-2-YLAMINE
- CAS Number:53051-97-1
- Molecular formula:C6H8N2S
- Molecular Weight:140.21
82514-58-7
42 suppliers
$28.60/250mg
53051-97-1
78 suppliers
$65.00/100mg
Yield:53051-97-1 85%
Reaction Conditions:
with sodium hydrogencarbonate in tetrahydrofuran;water;ethyl acetate at 20; for 0.25 h;
Steps:
4
Example 4; 5,6-Dihydro-4H-cyclopentathiazol-2-ylamine (2), free base: The hydrochloride salt of 2 (16.5 g, 93 mmol) (for synthesis of HCl salt, see, e.g., Erlenmeyer and Scheonauer, Helv. Chim. Acta, vol. 24, p. 172E and 175 (1941) and Huenig et al., Chem. Ber., vol. 93, p. 1518-1525 (1960)) was added to a mixture of aqueous sodium bicarbonate (16.5 g, 250 mL) and 25% THF/EtOAc (160 mL, v/v). The mixture was stirred at room temperature for 15 min. The mixture was clarified by filtration through a pad of celite. The organic layer was separated, dried (MgSO4), and concentrated in vacuo to 47 g. Toluene (100 mL) was added and the mixture was concentrated in vacuo to 45 g. The mixture was diluted with heptane (40 mL), cooled, and the product was collected by filtration and dried to give the title compound as the free base (10.75 g, 85%). 1H NMR (CD3OD) δ 4.88 (2H, s), 2.75-2.70 (2H, m), 2.60-2.55 (2H, m), 2.39-2.33 (2H, m).
References:
US2007/149499,2007,A1 Location in patent:Page/Page column 19

17356-08-0
3 suppliers
inquiry

120-92-3
401 suppliers
$11.00/25g
53051-97-1
78 suppliers
$65.00/100mg
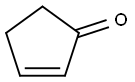
930-30-3
261 suppliers
$10.00/1g

17356-08-0
3 suppliers
inquiry
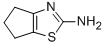
53051-97-1
78 suppliers
$65.00/100mg
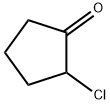
694-28-0
118 suppliers
$30.00/1g

17356-08-0
3 suppliers
inquiry
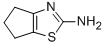
53051-97-1
78 suppliers
$65.00/100mg

17356-08-0
3 suppliers
inquiry

1056246-36-6
1 suppliers
inquiry
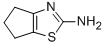
53051-97-1
78 suppliers
$65.00/100mg