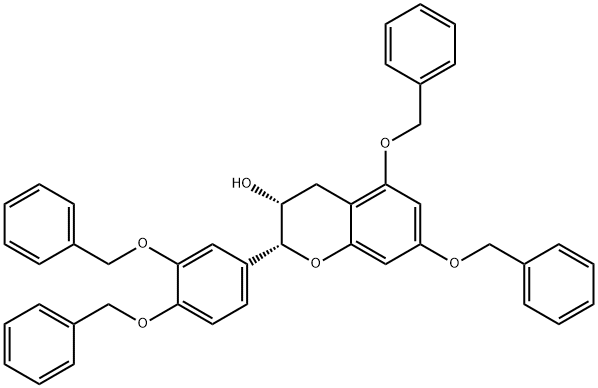
5,7,3'',4''-Tetra-O-benzylepicatechin synthesis
- Product Name:5,7,3'',4''-Tetra-O-benzylepicatechin
- CAS Number:87292-49-7
- Molecular formula:C43H38O6
- Molecular Weight:650.76
![2H-1-Benzopyran-3(4H)-one, 2-[3,4-bis(phenylmethoxy)phenyl]-5,7-bis(phenylmethoxy)-, (2R)-](/CAS/20210305/GIF/87292-54-4.gif)
87292-54-4
0 suppliers
inquiry

87292-49-7
2 suppliers
inquiry
Yield:87292-49-7 81%
Reaction Conditions:
Stage #1: (+)-(2R)-5,7-bis(benzyloxy)-2-[3,4-bis(benzyloxy)phenyl]chroman-3-onewith L-Selectride;lithium bromide in tetrahydrofuran at -78; for 3.08333 h;
Stage #2: with sodium hydroxide;dihydrogen peroxide in tetrahydrofuran;ethanol;water at 20;
Steps:
1.C
A 1 M solution of lithium tri-sec-butylborohydride in tetrahydrofuran, herein after THF, (100 mL, L-Selectride, sold by the Aldrich Chemical Co, Inc., Milwaukee, Wis.) was added, under an argon atmosphere, to a stirred, 0° C. solution of anhydrous lithium bromide, LiBr, (34.9 g, 402 mmol) in 100 mL anhydrous THF. The resulting mixture was cooled to -78° C., using an acetone/CO2 bath, followed by dropwise addition of a solution of the flavanone according to Part B (50.1 g, 77.2 mmol) in 400 mL of anhydrous THF, over a period of 50 min. Stirring was continued at -78° C. for 135 min. The cooling bath was removed and 360 mL of 2.5 M aqueous sodium hydroxide (NaOH) was added to the reaction mixture. The reaction flask was placed in a room temperature water bath and a mixture of 35% aqueous H2O2 (90 mL) and ethanol (270 mL) was added over a period of 130 min. Stirring was continued overnight. Chloroform (700 mL) was added to dissolve the crystallized product, the phases were separated, the aqueous phase was extracted with CHCl3 (50 mL), the combined organic phases were dried over MgSO4, evaporated and dried in vacuo to provide 56.6 g of crude product. This material was dissolved in 600 mL of a boiling mixture of ethyl acetate (EtOAc) and ethanol (EtOH) (2:3), and allowed to crystallize at room temperature, then in the refrigerator. The product was isolated by suction filtration, washed with 2×50 mL of cold (-20° C.) EtOAc/EtOH (1:3), and dried in vacuo first at room temperature, then at 80° C. to obtain 35.4 g (70%) of a light yellow solid. The evaporated mother liquor was filtered over silica gel, SiO2, (14×6.5 cm, CHCl3, then CHCl3/EtOAc 12:1), the eluate concentrated to 40 mL, and the residue diluted with 60 mL of ethanol, to obtain an additional 5.5 g (11%) of the O-benzylepicatechin as a yellowish solid: mp 129.5-130° C. (from EtOAc/EtOH); [α]D-27.7° [α]546-33.4° (EtOAc, c 21.6 g/L); 1H NMR (CDCl3) δ 7.48-7.25 (m, 20 H), 7.14 (s, 1 H), 7.00, 6.97 (ABq, 2 H. J=8.5 Hz, A part d with J=1.5 Hz), 6.27 (s, 2 H), 5.19 (s, 2 H), 5.18 (s, 2 H), 5.02 (s, 2 H), 5.01 (s, 2 H), 4.91 (s, 1 H), 4.21 (br s, 1 H), 3.00, 2.92 (ABq, 2 H, J=17.5 Hz, both parts d with J=1.5 and 4 Hz, resp.), 1.66 (d, 1 H, J=5.5 Hz); Anal. Calcd. for C43H38O6: C, 79.36; H, 5.89. Found: C, 79.12: H, 5.99.
References:
US2005/277600,2005,A1 Location in patent:Page/Page column 12-13
![Benzeneacetic acid, α-methoxy-, (2R,3R)-2-[3,4-bis(phenylmethoxy)phenyl]-3,4-dihydro-5,7-bis(phenylmethoxy)-2H-1-benzopyran-3-yl ester, (αS)-](/CAS/20210305/GIF/1392108-92-7.gif)
1392108-92-7
0 suppliers
inquiry

87292-49-7
2 suppliers
inquiry
![(2R-trans)-2-[3,4-bis(phenylmethoxy)phenyl]-3,4-dihydro-5,7-bis(phenylmethoxy)-2H-1-benzopyran-3-ol](/CAS/GIF/20728-73-8.gif)
20728-73-8
12 suppliers
$165.00/100mg

87292-49-7
2 suppliers
inquiry

