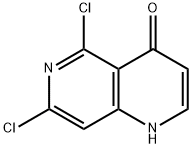
5,7-DICHLORO-1H-[1,6]NAPHTHYRIDIN-4-ONE synthesis
- Product Name:5,7-DICHLORO-1H-[1,6]NAPHTHYRIDIN-4-ONE
- CAS Number:863785-66-4
- Molecular formula:C8H4Cl2N2O
- Molecular Weight:215.04
![5-{[(2,6-dichloropyridin-4-yl)amino]methylidene}-2,2-dimethyl-1,3-dioxane-4,6-dione](/CAS/20210111/GIF/863785-65-3.gif)
863785-65-3
4 suppliers
inquiry
![5,7-DICHLORO-1H-[1,6]NAPHTHYRIDIN-4-ONE](/CAS/GIF/863785-66-4.gif)
863785-66-4
29 suppliers
$45.00/10mg
Yield:863785-66-4 94%
Reaction Conditions:
in diphenylether at 220; for 1 h;
Steps:
5 5,7-dichloro-1,6-naphthyridin-4(1H)-one (51)
2,6-dichloro-4-aminopyridine (1 g, 6.13 mmol) was dissolved in isopropanol, then 2 eq of compound 49 was added thereto, and then the mixture was warmed to 110 and reacted for 1 h with stirring, then a large amount of solid was precipitated.
The reaction was continued for another 2 h with stirring, then the resultant was cooled to room temperature, washed with isopropanol and ether to give the product, yield 96%;
The compound prepared as above was suspended in diphenyl ether at 10 ml/g, warmed to 220° C. and reacted for 1 h with stirring, and then a large amount of solid was precipitated, which was cooled to room temperature, washed with a large amount of PE and filtered to give the product, yield 94%. 1H NMR (300 MHz, DMSO) δ 12.11 (s, 1H), 7.93 (dd, J=7.7, 5.5 Hz, 1H), 7.48 (s, 1H), 6.17 (d, J=7.5 Hz, 1H).
References:
US2018/244667,2018,A1 Location in patent:Paragraph 0095-0096
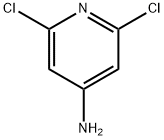
2587-02-2
314 suppliers
$12.40/250mg
![5,7-DICHLORO-1H-[1,6]NAPHTHYRIDIN-4-ONE](/CAS/GIF/863785-66-4.gif)
863785-66-4
29 suppliers
$45.00/10mg