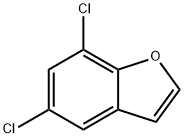
5,7-Dichlorobenzofuran synthesis
- Product Name:5,7-Dichlorobenzofuran
- CAS Number:23145-06-4
- Molecular formula:C8H4Cl2O
- Molecular Weight:187.02
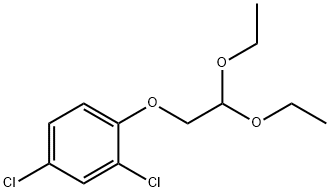
78830-79-2
10 suppliers
$28.60/50MG

23145-06-4
19 suppliers
inquiry
Yield:23145-06-4 30%
Reaction Conditions:
with tin-exchanged H-b zeolite (Sn-b) for 10 h;Reflux;
Steps:
3.3. General procedure for the synthesis of 2,3-unsubstitutedbenzo[b]furans
General procedure: A 25 mL round-bottomed flask was charged with 2-aryloxyacetaldehyde diethyl acetals (1 mmol), Sn-b (0.1 g), andtrifluorotoluene (10 mL). The mixture was stirred under refluxingcondition and monitored by GC. Upon completion, the mixture wascooled to room temperature, and the catalyst Sn-b was filtrate off.The filter cake was washed with trifluorotoluene (10 mL3). Thecombined filtratewas concentrated under vacuum. The residuewaspurified by flash column chromatography on SiO2 (petroleumether/ethyl acetate) to afford the desired 2,3-unsubstituted benzo[b]furans.
References:
Sun, Nan;Huang, Peng;Wang, Yifan;Mo, Weimin;Hu, Baoxiang;Shen, Zhenlu;Hu, Xinquan [Tetrahedron,2015,vol. 71,# 29,p. 4835 - 4841]
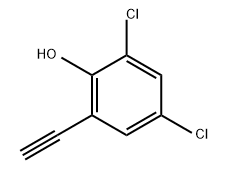
936840-15-2
1 suppliers
inquiry

23145-06-4
19 suppliers
inquiry

2040-83-7
25 suppliers
inquiry

23145-06-4
19 suppliers
inquiry

120-83-2
400 suppliers
$12.90/100g

23145-06-4
19 suppliers
inquiry