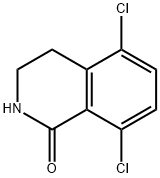
5,8-dichloro-3,4-dihydroisoquinolin-1(2H)-one synthesis
- Product Name:5,8-dichloro-3,4-dihydroisoquinolin-1(2H)-one
- CAS Number:1616289-34-9
- Molecular formula:C9H7Cl2NO
- Molecular Weight:216.06
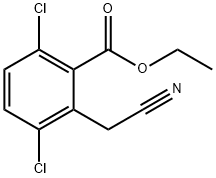
1616289-32-7
4 suppliers
inquiry

1616289-34-9
16 suppliers
inquiry
Yield:1616289-34-9 29.3 g
Reaction Conditions:
with sodium tetrahydroborate;cobalt(II) chloride hexahydrate in ethanol at 0 - 20;Reflux;
Steps:
253
Cobalt (II) chloride hexahydrate (166 g, 0.70 mol) was added to a room temperature solution of ethyl 3,6-dichloro-2-(cyanomethyl)benzoate (253d, 90 g, 0.35 mol) in ethanol (1.5 L), and the resulting mixture cooled to 0° C. Sodium borohydride (66.3 g, 1.74 mol) was added in portions.
The mixture was stirred at room temperature for 1 hour, and then refluxed overnight.
The resulting suspension was filtered and the filtrate concentrated in vacuo.
The solids in the filter cake were stirred in ethyl acetate (600 mL), and then filtered again.
This procedure was repeated a second time.
The combined filtrates were added to the original filtrate residue, and this organic solution washed with water (800 mL) and saturated aqueous sodium chloride solution (800 mL), dried over sodium sulfate, and concentrated in vacuo to give 5,8-dichloro-3,4-dihydroisoquinolin-1(2H)-one (253e, 29.3 g, 39% yield) as an off-white solid.
References:
US2014/179667,2014,A1 Location in patent:Paragraph 0857; 0861

1616289-31-6
6 suppliers
inquiry

1616289-34-9
16 suppliers
inquiry
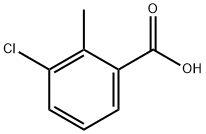
7499-08-3
174 suppliers
$8.00/10g

1616289-34-9
16 suppliers
inquiry

1616289-30-5
5 suppliers
inquiry

1616289-34-9
16 suppliers
inquiry
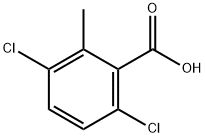
1918-01-0
6 suppliers
inquiry

1616289-34-9
16 suppliers
inquiry