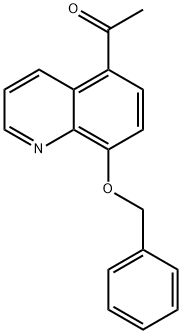
5-Acetyl-8-(phenylMethoxy)quinoline synthesis
- Product Name:5-Acetyl-8-(phenylMethoxy)quinoline
- CAS Number:26872-48-0
- Molecular formula:C18H15NO2
- Molecular Weight:277.32
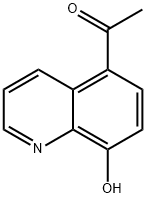
2598-31-4
55 suppliers
$160.00/250mg

100-39-0
437 suppliers
$10.00/10g

26872-48-0
12 suppliers
$150.00/25mg
Yield:-
Reaction Conditions:
with potassium carbonate in acetone; for 14 h;Heating / reflux;
Steps:
2
Example 2Preparation of 5-acetyl-8-benzyloxyquinoline (compound (Ic); Ri = benzyl)
References:
WO2008/104781,2008,A1 Location in patent:Page/Page column 37-38
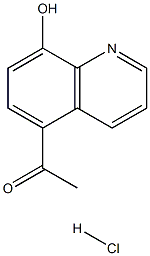
57434-96-5
0 suppliers
inquiry

100-44-7
655 suppliers
$13.50/250G

26872-48-0
12 suppliers
$150.00/25mg
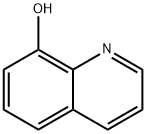
148-24-3
850 suppliers
$5.00/25g

26872-48-0
12 suppliers
$150.00/25mg

57434-96-5
0 suppliers
inquiry

26872-48-0
12 suppliers
$150.00/25mg