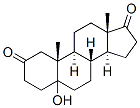
5-ALPHA-ANDROSTANE-ALPHA-NOR-2,17-DIONE synthesis
- Product Name:5-ALPHA-ANDROSTANE-ALPHA-NOR-2,17-DIONE
- CAS Number:1032-12-8
- Molecular formula:
- Molecular Weight:0
481-29-8
350 suppliers
$39.00/5mg

1032-12-8
3 suppliers
inquiry
Yield:1032-12-8 18 g
Reaction Conditions:
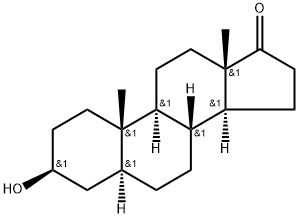
Stage #1: 3-hydroxy-5α-androstane-17-onewith Jones reagent;acetic acid at 55 - 90; for 2 h;Inert atmosphere;
Stage #2: with sodium acetate;acetic anhydride for 4 h;Reflux;
Steps:
1.I.I; 1.I.II
Reaction step I: Preparation of Jones reagent: 57g CrO3was soluted in 400ml H2O, and then slowly added 85ml H2SO4. The yielding Jones reagent was then colded to 40. 50g of 5α-androstane and 200ml of acetic acid were added into a 1L three-necked flask. The mixture was warmed to 55-60, stirred and dissolved under nitrogen atmosphere. Jones reagent was added dropwise within 1hour. Reaction temperature was increased to 90 and reacted for 1 hour, and acetic acid was distilled the reduced pressure. The residue was filtrated, washed with H2O and dryed to give 50g of di-acidic product (b).; Reaction step II: 30 g of di-acid product (b) and 600 mL of acetic anhydride were dissolved in 1L three-necked flask and added in 24 g sodium acetate, stirred and refluxed for 4 hours. The residue was filtrated and dried and give 18 g of di-ketone product (c) .
References:
WO2019/42192,2019,A1 Location in patent:Paragraph 00152-00154
5226-04-0
0 suppliers
inquiry

1032-12-8
3 suppliers
inquiry
![Dicyclopenta[a,f]naphthalen-2(1H)-one, tetradecahydro-6-hydroxy-3a,5a-dimethyl-, (3aS,3bS,5aS,6S,8aS,8bR,10aS)-](/CAS/20210305/GIF/1032-10-6.gif)
1032-10-6
0 suppliers
inquiry

1032-12-8
3 suppliers
inquiry

6242-26-8
1 suppliers
inquiry

1032-12-8
3 suppliers
inquiry
![1H-Benz[e]indene-6,7-diacetic acid, dodecahydro-3-hydroxy-3a,6-dimethyl-, (3S,3aS,5aS,6S,7S,9aR,9bS)-](/CAS/20210305/GIF/1046-34-0.gif)
1046-34-0
0 suppliers
inquiry

1032-12-8
3 suppliers
inquiry