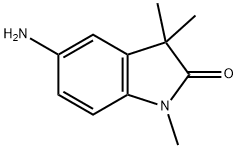
5-Amino-1,3,3-trimethyl-2-oxindole synthesis
- Product Name:5-Amino-1,3,3-trimethyl-2-oxindole
- CAS Number:953048-71-0
- Molecular formula:C11H14N2O
- Molecular Weight:190.24
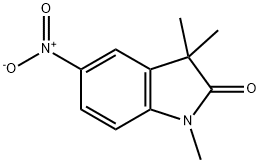
120791-55-1
8 suppliers
inquiry

953048-71-0
29 suppliers
$90.00/100mg
Yield:953048-71-0 93%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethanol; under 760.051 Torr; for 48 h;
Steps:
2A 5-Amino-1,3,3-trimethyl-1,3-dihydro-2H-indol-2-one
2.32 g (10.56 mmol) of 1,3,3-trimethyl-5-nitro-1,3-dihydro-2H-indol-2-one from Example 1A were initially charged in 71.5 ml of ethanol, 330 mg (0.32 mmol) of palladium (10% on activated carbon) were added and the mixture was hydrogenated at hydrogen standard pressure for 2 days.
Subsequently, the reaction mixture was filtered through kieselguhr, the residue was washed with ethanol and the filtrate was concentrated.
The residue was stirred with a little ethanol, filtered off, washed with a little ethanol, filtered off with suction and dried.
This gave 1.95 g (93% of theory) of the title compound.
LC-MS (Method 4): Rt=0.76 min; MS (ESIpos): m/z=191 (M+H)+.
1H-NMR (400 MHz, DMSO-d6): δ [ppm]=1.20 (s, 6H), 3.04 (s, 3H), 4.70-4.80 (m, 2H), 6.46 (dd, 1H), 6.58 (d, 1H), 6.67 (d, 1H).
References:
US2016/244415,2016,A1 Location in patent:Paragraph 0605; 0606; 0607; 0608
20200-86-6
14 suppliers
inquiry

953048-71-0
29 suppliers
$90.00/100mg