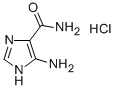
5-AMINO-1-PROPYL-1H-IMIDAZOLE-4-CARBOXAMIDE synthesis
- Product Name:5-AMINO-1-PROPYL-1H-IMIDAZOLE-4-CARBOXAMIDE
- CAS Number:61507-88-8
- Molecular formula:C7H12N4O
- Molecular Weight:168.2
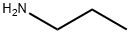
107-10-8
354 suppliers
$10.00/20mg
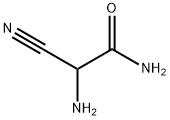
6719-21-7
115 suppliers
$35.00/500mg
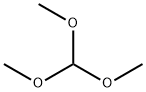
149-73-5
521 suppliers
$12.00/5g

61507-88-8
9 suppliers
$220.00/1g
Yield:61507-88-8 61%
Reaction Conditions:
in acetonitrile;
Steps:
2 5-Amino-1-n-propylimidazole-4-carboxamide
PREPARATION 2 5-Amino-1-n-propylimidazole-4-carboxamide A mixture of 2-amino-2-cyanoacetamide (2.8 g, 0.0282 mol), trimethylorthoformate (3.4 g, 0.0321 mol) and acetonitrile (55 ml) was heated under reflux for 0.75 hour, then allowed to cool. n-Propylamine (1.8 g, 0.0305 mol) was then added dropwise, and the resulting mixture stirred at ambient temperature for 36 hours. The solid which precipitated was collected by filtration and crystallized from methanol to give the title compound as a colourless solid (2.9 g, 61%), m.p. 241°-243° C. Found: C,49.67; H,7.15; N,33.64. C7 H12 N4 O requires C,49.98; H,7.19; N,33.31%.
References:
US5734053,1998,A
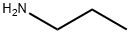
107-10-8
354 suppliers
$10.00/20mg
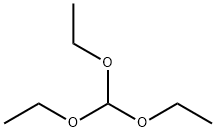
122-51-0
486 suppliers
$10.00/5ml

6719-21-7
115 suppliers
$35.00/500mg

61507-88-8
9 suppliers
$220.00/1g