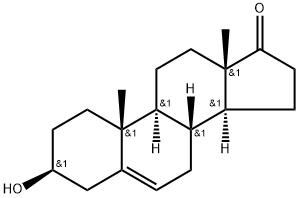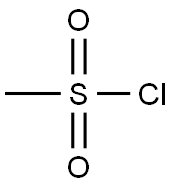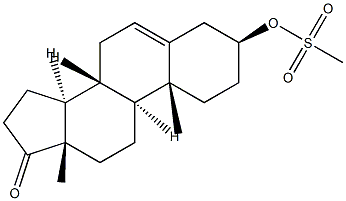
5-Androsten-3β-ol-17-one Methanesulfonate synthesis
- Product Name:5-Androsten-3β-ol-17-one Methanesulfonate
- CAS Number:25810-70-2
- Molecular formula:C20H30O4S
- Molecular Weight:366.51
Yield:25810-70-2 98.4%
Reaction Conditions:
with pyridine at 20 - 25; for 20 h;Inert atmosphere;
Steps:
3.1
1 In a 100 mL single-mouth flask, add 5.76 g of C-1 (20 mmol) and 20 mL of pyridine.Stir at room temperature, then add 1.86 mL of methylsulfonyl chloride (24 mmol) and react under nitrogen for 20 h at 20-25 °C.After the TLC detects the completion of the reaction of the starting material, the reaction is stopped.The reaction solution was poured into a dilute aqueous hydrochloric acid solution (25 mL of concentrated hydrochloric acid + 450 mL of water) and stirred at room temperature for 1 h.Filtered, the solid was rinsed with water (40mL×5).Drying gave 7.2 g of product C-2 as an off white solid. yield: 98.4%
References:
CN108864237,2018,A Location in patent:Paragraph 0044; 0045; 0046