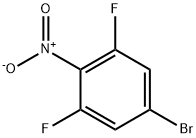
5-Bromo-1,3-difluoro-2-nitrobenzene synthesis
- Product Name:5-Bromo-1,3-difluoro-2-nitrobenzene
- CAS Number:147808-42-2
- Molecular formula:C6H2BrF2NO2
- Molecular Weight:237.99
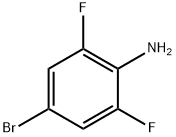
67567-26-4
347 suppliers
$5.00/1g

147808-42-2
171 suppliers
$5.00/250mg
Yield:147808-42-2 68%
Reaction Conditions:
Stage #1:4-bromo-2,6-difluoroaniline with sodium perborate in acetic acid at 65 - 70; for 72 h;Heating / reflux;
Stage #2: with water at 0;
Steps:
24
To a suspension of sodium perborate tetrahydrate (1.78 mmol, 0.27 g) in 10 ml of acetic acid stirred at 650C was added a solution of (4-bromo-2,6-difluorophenyl)amine (1.78 mmol, 0.42 g, 1 eq.) in 5 ml of acetic acid over one hour dropwise. The reaction was heated at 650C over 3 days. Sodium perborate tetrahydrate (2 eq., 3.56 mmol, 0.54 g) was added again. After 3 hours, sodium perborate tetrahydrate (1eq., 1.78 mmol, 0.27 g) was added again. Additional sodium perborate tetrahydrate (3eq., 5.34 mmol, 0.81 g) was again added. The reaction was then left overnight at 7O0C under reflux and under argon. The solution was then cooled to room temperature and poured onto ice and extracted with ethyl acetate (2x). The combined organics were washed with water and brine. The organics were dried over MgSO4, filtered and the organic solvent was removed under reduced pressure to afford the crude product which was purified by silica chromatography (ethyl acetate-4% n-hexane 96%) to afford the title compound, 0.29 g, 68%. 1H NMR δ (d6DMSO, 400MHz): 7.964 (2H, d)
References:
GLAXO GROUP LIMITED WO2007/36715, 2007, A2 Location in patent:Page/Page column 51
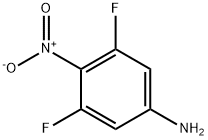
122129-79-7
52 suppliers
inquiry

147808-42-2
171 suppliers
$5.00/250mg
5509-65-9
481 suppliers
$10.00/10g

147808-42-2
171 suppliers
$5.00/250mg