
5-BroMo-1H-indole-3-triMethyl ethanone synthesis
- Product Name:5-BroMo-1H-indole-3-triMethyl ethanone
- CAS Number:32387-18-1
- Molecular formula:C10H5BrF3NO
- Molecular Weight:292.05
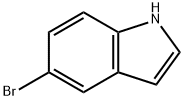
10075-50-0
689 suppliers
$5.00/10g
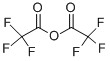
407-25-0
470 suppliers
$9.00/1g

32387-18-1
38 suppliers
$41.00/250mg
Yield:32387-18-1 98%
Reaction Conditions:
in N,N-dimethyl-formamide at 0; for 2.5 h;Inert atmosphere;
Steps:
1.1 1-(5-bromo-1H-indol-3-yl)-2,2,2-trifluoroethanone (hb)
In a dry flask under argon, 5-bromoindole (ab) (3.0 g, 15.31 mmol) was dissolved in dry DMF (15 mL). This solution was cooled to 0 °C and trifluoroacetic anhydride (3.19 mL, 22.96 mmol) was added dropwise. The mixture was stirred at 0 °C for 2h30 then quenched with water. The crude mixture was filtered to afford a solid. This solid was washed twice with water and dissolved in ethyl acetate. The organic layer was washed with an aqueous solution of NaHCO3 and brine, dried over anhydrous MgSO4 and evaporated under vacuum. The desired product (hb) was obtained as a solid (4.381 g, 15.0 mmol) without further purification. Yield: 98%. 1H NMR (300 MHz, CDCl3): δ = 7.35 (d, J = 8.4 Hz, 1H, CH), 7.48 (dd, J = 2.4 and 8.4 Hz, 1H, CH), 8.06 (d, J = 1.8 Hz, 1H, CH), 8.58 (d, J = 2.1 Hz, 1H, CH), 8.90 (br s, 1H, NH) ppm. LRMS (ESI): m/z = 290 and 292 [(M-H)-].
References:
EP2548864,2013,A1 Location in patent:Paragraph 0145

10075-50-0
689 suppliers
$5.00/10g

32387-18-1
38 suppliers
$41.00/250mg