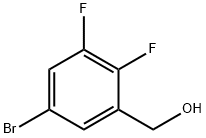
(5-bromo-2,3-difluorophenyl)methanol synthesis
- Product Name:(5-bromo-2,3-difluorophenyl)methanol
- CAS Number:887585-71-9
- Molecular formula:C7H5BrF2O
- Molecular Weight:223.01

633327-22-7
73 suppliers
$10.00/100mg

887585-71-9
25 suppliers
$105.00/100mg
Yield:887585-71-9 87%
Reaction Conditions:
with sodium tetrahydroborate in methanol at 20; for 1 h;Inert atmosphere;
Steps:
5.1
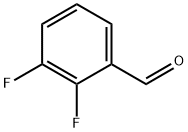
Step 1 followed the general procedure from Example 3 step 1. 2,3-difluorobenzaldehyde (200 mg, 1.408 mmol) was dissolved in concentrated H2S04 (0.64 mL) and heated to 60 00. To this was added N-bromosuccinimide (301 mg, 1.690 mmol) in three portions over a period of 20 mm. After being heated for 3 h under argon, the reaction mixture was poured into ice water. The product was extracted with hexanes, washed with water and brine, and then dried over anhydrous Na2SO4. Purification by flash chromatography yielded an orange liquid as 2,3- difluoro-5-bromobenzaldehyde (155 mg, 50% yield), which was dissolved in 6 mL of methanol. To this stirring solution was added NaBH4 (32 mg, 0.84 mmol). The reaction was stirred for Iat room temperature. Then the reaction was diluted with ethyl acetate, washed with saturated aqueous NH4CI, followed by brine. The organic layer was dried over anhydrous Na2SO4, and evaporated under reduced pressure yielded 2,3-difluoro-5-bromobenzyl alcohol as a white solid (163 mg, 87% yield). The boronate product was then obtained from 2,3-difluoro-5-bromobenzyl alcohol according to the general procedure of Example 1. The crude material was used for Suzuki coupling reaction without purification.
References:
WO2016/118825,2016,A1 Location in patent:Page/Page column 32
2646-91-5
288 suppliers
$5.00/250mg

887585-71-9
25 suppliers
$105.00/100mg