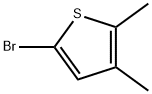
5-bromo-2,3-dimethylthiophene synthesis
- Product Name:5-bromo-2,3-dimethylthiophene
- CAS Number:172319-78-7
- Molecular formula:C6H7BrS
- Molecular Weight:191.09
632-16-6
113 suppliers
$13.00/1g

172319-78-7
13 suppliers
inquiry
Yield:172319-78-7 52%
Reaction Conditions:
with N-Bromosuccinimide in acetic acid at 50; for 0.25 h;
Steps:
5 Synthesis of 5-bromo-2,3-dimethylthiophene (8)
To a solution of 2,3-dimethylthiophene (21.68 g, 192 mmol) in glacial acetic acid (20 mL) was added N-bromosuccinimide (NBS) (34.39 g, 192 mg) over 5 minutes (the temperature increased to 50° C.). The reaction was complete (TLC: hexanes) after 10 minutes then poured over ice. Once cooled, the organics were extracted with DCM and the combined fractions were washed with 1M NaOH, water and brine. The resulting solution was dried with MgSO4, concentrated under vacuum to yield a light orange oil. Flash chromatography afforded 8 (19.07 g, 52%). 1H NMR (600 MHz, CDCl3) δ 6.72 (s, 1H), 2.27 (s, 3H), 2.09 (s, 3H).
References:
US2014/256936,2014,A1 Location in patent:Paragraph 0221