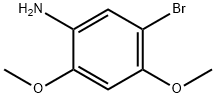
5-Bromo-2,4-dimethoxyaniline synthesis
- Product Name:5-Bromo-2,4-dimethoxyaniline
- CAS Number:169883-36-7
- Molecular formula:C8H10BrNO2
- Molecular Weight:232.07
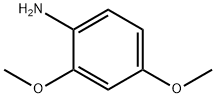
2735-04-8
150 suppliers
$24.00/25g

169883-36-7
22 suppliers
inquiry
Yield: 23%
Reaction Conditions:
with bromine;potassium carbonate in chloroform for 4 h;
Steps:
A.75 Example 75; N-(5-bromo-2,4-dimethoxyphenyl)-N'-(5-methylisoxazol-3-yl)urea.
Bromine (10.6 g, 66.3 mmol) dissolved in 40 mL CHCl3 is added dropwise to a mixture of K2CO3 (20.8 g, 150.5 mmol) and 2,4-dimethoxyaniline (10.0 g, 65.3 mmol) dissolved in 60 mL CHC3. After stirring the mixture for 4 h, water is added. The layers are separated and the organic layer is washed with water, dried (MgSO4), filtered and concentrated. The residue is purified by column chromatography (Biotage Flash 40M column, 20% EtOAc/hexanes) and recrystallized from hexanes to provide 3.41 g (23% yield) of 5-bromo-2,4-dimethoxyaniline. MS for C8H10BrNO2 (ESI) (M+H)+ m/z 232 and 234. An EtOAc solution of 5-bromo-2,4-dimethoxyaniline (0.822 g, 3.54 mmol) is added to excess phosgene (5.0 mL, 20% solution in toluene). The solution is heated under reflux for 30 min, cooled and concentrated in vacuo. The residue is recrystallized from heptane to provide 0.81 g (89% yield) of 1-bromo-5-isocyanato-2,4-dimethoxybenzene. Example 75 is obtained from 1-bromo-5-isocyanato-2,4-dimethoxybenzene according to Method A, making non-critical variations. Yield 40%. HRMS (FAB) calculated for C13H14BrN3O4+H 356.0246, found 356.0256.
References:
Piotrowski, David W.;Rogers, Bruce N.;McWhorter JR., William W.;Walker, Daniel Patrick;Corbett, Jeffrey W.;Groppi JR., Vincent E.;Rudmann, Daniel G. US2003/236287, 2003, A1 Location in patent:Page 28, 29
778599-56-7
4 suppliers
inquiry

169883-36-7
22 suppliers
inquiry

2735-04-8
150 suppliers
$24.00/25g

169883-36-7
22 suppliers
inquiry
4920-84-7
136 suppliers
$12.00/1g

169883-36-7
22 suppliers
inquiry
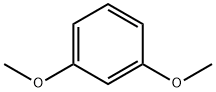
151-10-0
448 suppliers
$5.00/10g

169883-36-7
22 suppliers
inquiry