
5-Bromo-2-[4-(trifluoromethoxy)phenyl]pyridine synthesis
- Product Name:5-Bromo-2-[4-(trifluoromethoxy)phenyl]pyridine
- CAS Number:1257875-15-2
- Molecular formula:C12H7BrF3NO
- Molecular Weight:318.09
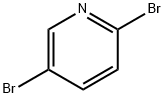
624-28-2
718 suppliers
$5.00/5g

139301-27-2
379 suppliers
$14.00/5G
![5-Bromo-2-[4-(trifluoromethoxy)phenyl]pyridine](/CAS/20180703/GIF/1257875-15-2.gif)
1257875-15-2
10 suppliers
inquiry
Yield:-
Reaction Conditions:
with caesium carbonate;bis-triphenylphosphine-palladium(II) chloride in ethanol;toluene at 80; for 1 h;Inert atmosphere;
Steps:
1
A mixture of 5.00 g (21.11 mmol) of 2,5-dibromopyridine (ALDRICH), 7.56 g (23.22 mmolo) of caesium carbonate and 4.13 g (20.05 mmol) of 4- (trifluoromethoxy)benzeneboronic acid (FRONTIER SCIENTIFIC) in 165 ml Tol/EtOH (10:1 ) was stirred and bubbled with argon for 15 min. To this mixture were added 0.741 g (1.055 mmol) of bis(triphenylphosphine)palladium(ll) chloride, it was bubbled with argon for another 15 min. The reaction was heated 1 h at 80 0C. The reaction mixture was quenched with water and extracted with AcOEt. Combined the organic layers were washed with saturated brine, dried over sodium sulfate and evaporated in vacuo. The residue obtained was purified by flash master (20 g, Sill, 15:1 Hex:AcOEt) to afford 5.8 g of the title compound.1H NMR (δ, ppm, DMSO-d6 ): 8.80 (d, 1 H); 8.25-8.10 (m, 3H); 7.99 (d, 1 H); 7.48 (d, 2H). [ES MS] m/z: 318 [M+H]+.
References:
WO2010/142741,2010,A1 Location in patent:Page/Page column 19; 20
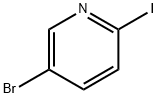
223463-13-6
372 suppliers
$5.00/1g

139301-27-2
379 suppliers
$14.00/5G
![5-Bromo-2-[4-(trifluoromethoxy)phenyl]pyridine](/CAS/20180703/GIF/1257875-15-2.gif)
1257875-15-2
10 suppliers
inquiry