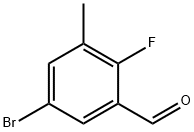
5-BROMO-2-FLUORO-3-METHYLBENZALDEHYDE synthesis
- Product Name:5-BROMO-2-FLUORO-3-METHYLBENZALDEHYDE
- CAS Number:903875-64-9
- Molecular formula:C8H6BrFO
- Molecular Weight:217.04
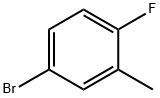
51437-00-4
273 suppliers
$6.00/5g
108-18-9
380 suppliers
$10.00/1g

903875-64-9
63 suppliers
$22.00/250mg
Yield:-
Reaction Conditions:
with hydrogenchloride;n-butyllithium in tetrahydrofuran;hexane;N,N-dimethyl-formamide
Steps:
96.1 Step 1.
Step 1.
5-Bromo-2-fluoro-3-methylbenzaldehyde
To a stirred solution of di-isopropyl amine (4.01 g, 39.88 mmol) in THF (20 mL) was added n-BuLi (1.6 M in hexane) (19.9 mL, 31.91 mmol) at -78° C. slowly dropwise over the period of 10 min, the reaction mixture was stirred at -78° C. for 30 min.
A solution of 4-bromo-1-fluoro-2-methylbenzene (5.0 g, 26.6 mmol) in THF (30.0 mL) was added at -78° C., and the reaction mixture was stirred for 1 h at the same temperature. DMF (5.0 mL) was added and stirred at -78° C. for another 30 min.
The reaction was monitored by TLC; then the reaction mixture was quenched with 1N HCl solution (aq) at 0° C.
The aqueous layer was extracted with Et2O, washed with water and saturated brine solution.
The combined organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure to obtain the crude compound purified by flash column chromatography (SiO2, 100-200 mesh; eluting with 5% EtOAc/pet ether) to afford the title compound as a white solid (3.6 g, 64%); mp 48-50° C.: 1H NMR (400 MHz, CDCl3) δ 8.33 (s, 1H), 8.22 (s, 1H), 7.67 (s, 1H), 7.60 (s, 1H), 6.75 (dd, J=17.6, 10.8 Hz, 1H), 5.92 (dd, J=17.6, 10.8 Hz, 1H), 5.52 (d, J=17.6 Hz, 1H), 2.21 (s, 3H); ESIMS m/z 211.35 ([M-H]-).
References:
Dow AgroSciences LLC;Lo, William C.;Hunter, James E.;Watson, Gerald B.;Patny, Akshay;Iyer, Pravin S.;Boruwa, Joshodeep US2014/171314, 2014, A1 Location in patent:Page/Page column