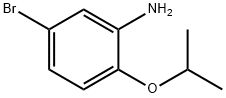
5-Bromo-2-isopropoxyaniline synthesis
- Product Name:5-Bromo-2-isopropoxyaniline
- CAS Number:1019442-22-8
- Molecular formula:C9H12BrNO
- Molecular Weight:230.1

383869-57-6
13 suppliers
inquiry

1019442-22-8
29 suppliers
inquiry
Yield:1019442-22-8 64%
Reaction Conditions:
with hydrogenchloride;tin(ll) chloride in tetrahydrofuran;water at 50; for 24 h;
Steps:
Bromo-isopropoxy amine (general procedure 3)
General procedure: To a solution of 4-bromo-1 -isopropoxy-2-nitrobenzene (prepared using general procedure 1 , 84%, 17.54 g, 67.4 mmol) in THF (270 mL), HCI (1 M aq, 270 mL, 270 mmol, 4.0 eq) and stannous chloride (38 g, 282 mmol, 3.0 eq) were added and heated to 50°C for 24 hours. Upon completion, the mixture was quenched with saturated aqueous sodium bicarbonate, filtered over celite, and the crude product was extracted twice with ethyl acetate. The combined organic layers were dried (Na2S04), concentrated, and purified by combiflash 0 to 30% EtOAc to yield bromo-isopropoxy amine (9.93 g, 64% yield) 1 H NMR (400 MHz, CDCI3): δ 6.82 p.p.m. (d, J=2.4 Hz, 1 H), 6.77 (dd, J1=8.5 Hz, J2=2.4 Hz, 1 H), 6.63 (d, J=8.6 Hz, 1 H), 4.46 (hept, J=1 .5 Hz, 1 H), 1 .33 (d, J=6.0, 6H), 13C NMR (125 MHz): δ 1 14.4, 138.9, 120.6, 1 17.6, 1 14.8, 1 13.2, 71 .0 HRMS (m/z): [M+] calculated for C9H13NOBr 230.1 , found 229.01
References:
WO2014/11973,2014,A2 Location in patent:Paragraph 0157; 0162