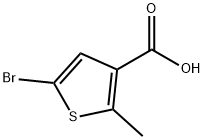
5-Bromo-2-methyl-thiophene-3-carboxylic acid synthesis
- Product Name:5-Bromo-2-methyl-thiophene-3-carboxylic acid
- CAS Number:1344027-40-2
- Molecular formula:C6H5BrO2S
- Molecular Weight:221.07
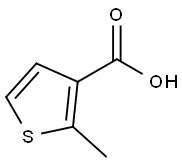
1918-78-1
83 suppliers
$9.00/250mg

1344027-40-2
20 suppliers
$110.00/50mg
Yield:1344027-40-2 84%
Reaction Conditions:
with acetic acid;pyridinium hydrobromide perbromide at 40; for 4 h;Inert atmosphere;
Steps:
4.2.7. Synthesis of 5-bromo-2-methylthiophene-3-carboxylic acid (8)
To a solution of 2-methylthiophene-3-carboxylic acid (2.44 g, 17.2 mmol, prepared from commercially available thiophene-3-carboxylic acid according to the known procedure (US 5840917 A1)) was added pyridium tribromide (6.3 g) in AcOH (30 mL) at room temperature. The mixture was stirred at 40 °C for 4 h. The mixture was cooled to room temperature and poured into H2O (350 mL) with stirring. The product was precipitated and the suspension was stirred at room temperature for 1 h. The precipitated solid was filtered, washed with H2O (500 mL) and dried under high vacuum at 45 °C. The crude product was used without further purification (3.18 g, 84%). 1H NMR (400 MHz, CDCl3) δ 7.38 (s, 1H), 2.70 (s, 3H); MS calcd for C6H5BrO2S (MW 221.07), found [M+H]+ 221.
References:
Lee, Suk Ho;Song, Kwang-Seop;Kim, Jong Yup;Kang, Misuk;Lee, Jun Sung;Cho, Seung-Hwan;Park, Hyun-Ju;Kim, Jeongmin;Lee, Jinhwa [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 19,p. 5813 - 5832] Location in patent:experimental part