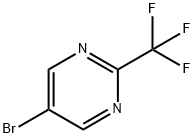
5-bromo-2-(trifluoromethyl)pyrimidine synthesis
- Product Name:5-bromo-2-(trifluoromethyl)pyrimidine
- CAS Number:799557-86-1
- Molecular formula:C5H2BrF3N2
- Molecular Weight:226.98
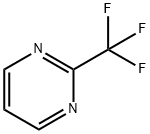
116470-67-8

799557-86-1
General procedure for the synthesis of 5-bromo-2-trifluoromethylpyrimidine using 2-trifluoromethylpyrimidine as starting material: 24 g of 2-trifluoromethylpyrimidine was dissolved in 200 ml of acetic acid and 50 g of bromine was added slowly and dropwise. The reaction mixture was heated to reflux and kept reacting overnight. After completion of the reaction, it was cooled to room temperature and extracted by adding appropriate amount of water and ethyl acetate. The organic and aqueous phases were separated and the organic phase was collected. The organic phase was dried with anhydrous sodium sulfate and subsequently concentrated under reduced pressure to give 29 g of 5-bromo-2-trifluoromethylpyrimidine.
680-15-9
252 suppliers
$13.00/5g
183438-24-6
389 suppliers
$6.00/1g

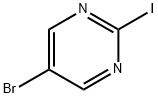
799557-86-1
166 suppliers
$57.00/500mg
Yield:799557-86-1 76.6%
Reaction Conditions:
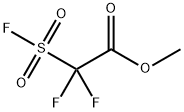
with copper(l) iodide in N,N-dimethyl-formamide at 80 - 90; for 3 h;
Steps:
1C.1
In step 1, DMF (149.6 kg, 7 L/kg compound 8A) was charged to an inerted reactor with stirring. Compound 8 A (22.5 kg, 78.98 moles, 1 equivalent) was charged to the reactor followed by Cul (6 kg, 0.4 eq., 2.674 kg/kg compound 8A) and followed by Me02CCF2S02F (21.2 kg, 0.944 kg/kg compound 8A). The reactor contents were stirred at 80- 90 °C for at least 3 hours to form a reaction product mixture comprising compound 8B. The reactor was sampled and tested for compound 8A content by LC with a limit of < 5% compound 8A. Mixing at 80-90 °C was continued until compound 8A content was < 5%. 25 molar NaHCCE (371.3 kg, 16.5 kg/kg compound 8A) was combined with the reaction product mixture and held for 6 hours with stirring at 20-30 °C. MTBE (116.6 kg, 5.18 kg/kg compound 8A) was combined with the reaction product mixture followed by celite filter aids (22.5 kg, 1 kg/kg compound 8A). The reaction product mixture was stirred for 15 minutes at 20-30 °C, filtered, and the filtrate was collected. The filter cake was washed with MTBE (33.3 kg, 1.48 kg/kg compound 8A) and the filtrates were combined.The collected filtrate was allowed to settle for at least 10 minutes and the bottom aqueous layer was removed. The aqueous layer was washed twice with MTBE (116.6 kg, 5.18 kg/kg compound 8A), where a phase separation and aqueous layer removal was done after each MTBE wash. The three organic layers were combined and further combined with 5 molar aqueous NaCl (212 kg, 9.42 kg/kg compound 8A) and stirred for at least 10 minutes. The admixture was allowed to settle for at least 10 minutes and the bottom aqueous layer was removed. The organic layer was combined with 5 molar aqueous NaCl (212 kg, 9.42 kg/kg compound 8A) and stirred for at least 10 minutes. The admixture was allowed to settle for at least 10 minutes and the bottom aqueous layer was removed. The organic layer remaining in the reactor was distilled under atmospheric pressure to reduce the volume to a minimum stir volume (about lL/kg compound 8A). The reactor contents were then further distilled under reduced pressure (50-100 mbar) and compound 8B was collected as an 80-90 °C fraction. The yield of compound 8A was 76.6% and the HPLC purity by method MTH-003 was 95.71% by HPLC Method 003 V01.
References:
WO2019/164778,2019,A1 Location in patent:Paragraph 0450; 0452-0454
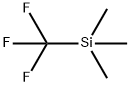
81290-20-2
417 suppliers
$13.00/1g

183438-24-6
389 suppliers
$6.00/1g

799557-86-1
166 suppliers
$57.00/500mg
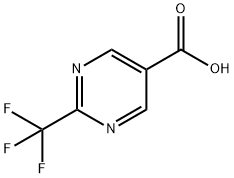
306960-77-0
157 suppliers
$33.00/250mg

799557-86-1
166 suppliers
$57.00/500mg

116470-67-8
82 suppliers
inquiry

799557-86-1
166 suppliers
$57.00/500mg