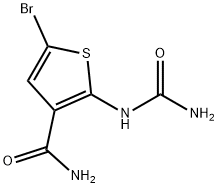
5-Bromo-2-ureidothiophene-3-carboxamide synthesis
- Product Name:5-Bromo-2-ureidothiophene-3-carboxamide
- CAS Number:354812-10-5
- Molecular formula:C6H6BrN3O2S
- Molecular Weight:264.1
![3-Thiophenecarboxamide, 2-[(aminocarbonyl)amino]-](/CAS/20210305/GIF/339365-14-9.gif)
339365-14-9
0 suppliers
inquiry

354812-10-5
9 suppliers
inquiry
Yield:354812-10-5 71%
Reaction Conditions:
with N-Bromosuccinimide in tetrahydrofuran at 0 - 20; for 16 h;Inert atmosphere;Darkness;
Steps:
1-(5-Bromo-3-carbamoylthiophen-2-yl)urea (8):
To a solution of 1-(3-carbamoylthiophen-2-yl)urea (140 mg, 0.756 mmol) in THF (9 mL) was added NBS (149 mg, 0.832 mmol) and the mixture was stirredat 0 °C for 1 h under shielding light. The reaction mixture was stirred at rt for 15 h, and concentrated. Purification by chromatography (20 g, CHCl3: MeOH = 10:1) gave compound 8 (140.9 mg, 0.534 mmol, 71%) as pink powder; mp 221-222 °C (decomposed); 1H NMR (400 MHz, CD3OD) δ 7.24 (s, 1H), 13C NMR (100 MHz, CD3OD) δ 168.6, 157.1, 151.5, 125.9, 113.4, 103.2; FT-IR (neat) 3352, 3193, 1702, 1546, 1511, 1420, 1328, 1275, 1220, 771, 694 cm-1; MS (FAB) m/z 91 (100), 263 [(M+H)+], 265[(M+H+2)+]; HRMS (FAB) calcd for C6H679BrN3O2S [(M+H)+] 263.9438, found 263.9440, calcd for C6H681BrN3O2S [(M+H+2)+] 265.9410, found 265.9416.
References:
Kawasaki, Norihiko;Fukuda, Hayato;Ishihara, Jun [Heterocycles,2020,vol. 101,# 2,p. 707 - 716]
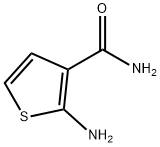
14080-51-4
137 suppliers
$8.00/250mg

354812-10-5
9 suppliers
inquiry