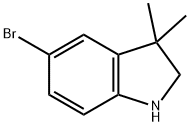
5-BroMo-3,3-diMethylindoline synthesis
- Product Name:5-BroMo-3,3-diMethylindoline
- CAS Number:53388-86-6
- Molecular formula:C10H12BrN
- Molecular Weight:226.11
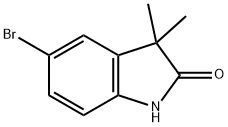
120902-45-6
59 suppliers
$65.00/50mg

53388-86-6
25 suppliers
inquiry
Yield:53388-86-6 87%
Reaction Conditions:
with TMAO in tetrahydrofuran;methanol;
Steps:
8 3,3-dimethyl-1,3-dihydro-2H-indol-2-one
To a solution of the 5-bromo-1,3-dihydro-3,3-dimethyl-2H-indol-2-one (0.9 g, 3.7 mmol) in 20 mL THF at 0° C. was added a borane-methylsulfide complex (2M in THF, 38 mL, 75 mmol). The reaction mixture was warmed to room temperature then brought to reflux for 4 hours. The mixture was cooled to room temperature and poured into H2O/CH2Cl2 and washed with 5% NaHCO3. The organic layer was washed with brine, dried (Na2SO4) and concentrated. The crude product was extracted in MeOH, trimethylamine N-oxide (2.0 g, 26.6 mmol) was added, and the solution was brought to reflux for 2 hours. The reaction mixture was cooled, concentrated and the crude residue purified by column chromatography (SiO2, methylene chloride) to afford 5-bromo-3,3-dimethyl-2,3-dihydro-1H-indole (0.074 g, 87%) as a yellow oil: 1H NMR (CDCl3) δ1.29 (s, 6H), 3.30 (s, 2H), 3.5 (br s, 1H), 6.49 (d, J=8.8 Hz, 1H), 7.08-7.12 (m, 2H); 13C-NMR (CDCl3) δ27.69 (q), 42.21 (s), 62.01 (d), 110.38 (s), 111.09, 125.46, 130.09 (d), 141.03, 149.52 (s); MS (EI) m/z 225, 227 (M)+.
References:
US6417214,2002,B1
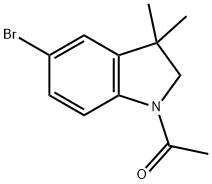
866364-70-7
0 suppliers
inquiry

53388-86-6
25 suppliers
inquiry
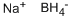
16940-66-2
8 suppliers
$23.30/100ml
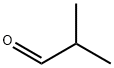
78-84-2
362 suppliers
$10.00/25mL
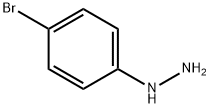
589-21-9
82 suppliers
inquiry

53388-86-6
25 suppliers
inquiry
