
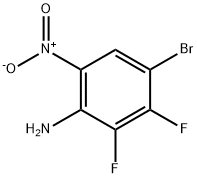
5-BroMo-3,4-difluorobenzene-1,2-diaMine synthesis
- Product Name:5-BroMo-3,4-difluorobenzene-1,2-diaMine
- CAS Number:1210048-11-5
- Molecular formula:C6H5BrF2N2
- Molecular Weight:223.02
626238-73-1
23 suppliers
$276.00/1g

1210048-11-5
12 suppliers
$318.00/250mg
Yield:1210048-11-5 87%
Reaction Conditions:
with tin(ll) chloride in ethanol at 70;
Steps:
96A
5.00 g (19.762 mmol) of the compound from example 95A was dissolved in 110 ml ethanol and 17.84 g (79.049 mmol) of tin(II) chloride dihydrate was added. The reaction solution was stirred overnight at 700C. After cooling, it was diluted with water and it was made weakly alkaline with saturated aqueous sodium hydrogen carbonate solution. The ethanol was removed from the mixture in a rotary evaporator, and the mixture was filtered on Celite and washed again with ethyl acetate. The organic phase was removed and the aqueous phase was extracted three times with ethyl acetate. The combined organic phases were dried over sodium sulfate and concentrated in a rotary evaporator. The residue was dried under high vacuum. We obtained 3.96 g (87% of theor.) of the target compound.LC-MS (method 10): R, = 0.81 min; MS (EIpos): m/z = 223 [M+H]+. 1H-NMR (400 MHz, DMSO-D6): δ [ppm] = 4.89 (sbr, 2H), 4.92 (sbr, 2H), 6.51 (dd, IH).
References:
WO2010/20363,2010,A1 Location in patent:Page/Page column 120-121

211693-73-1
103 suppliers
$5.00/250mg

1210048-11-5
12 suppliers
$318.00/250mg