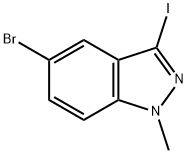
5-bromo-3-iodo-1-methyl-1H-indazole synthesis
- Product Name:5-bromo-3-iodo-1-methyl-1H-indazole
- CAS Number:865156-34-9
- Molecular formula:C8H6BrIN2
- Molecular Weight:336.96
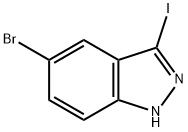
459133-66-5
150 suppliers
$10.00/250mg

74-88-4
341 suppliers
$15.00/10g

865156-34-9
18 suppliers
inquiry
Yield:865156-34-9 81 %
Reaction Conditions:
with caesium carbonate in acetonitrile at 20;
Steps:
66.1 Preparation of 5-bromo-3-iodo-1-methyl-1H-indazole
Step1:
Preparation of 5-bromo-3-iodo-1-methyl-1H-indazole. 5-bromo-3-iodo-1H-indazole (2.0 g, 6.2 mmol, 1.0 eq.) was dissolved in acetonitrile (30 mL), CH3I (1.3 g, 9.3 mmol, 1.5 eq.) and Cs2CO3 (3.0 g, 9.3 mmol, 1.5 eq.) were added, and the reaction mixture was stirred at room temperature for 1 h.
The solid was filtered out, the filtrate was rotary evaporated to dryness, and the crude product was purified by column chromatography (PE/EtOAc = 5:1-2:1, gradient elution) to give the title compound as a white solid (1.7 g, yield of 81%). MS (m/z) = 336.88 [M+H]+.
References:
EP4140996,2023,A1 Location in patent:Paragraph 0171; 0172
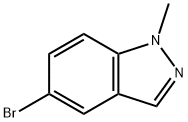
465529-57-1
150 suppliers
$20.00/1g

865156-34-9
18 suppliers
inquiry

459133-66-5
150 suppliers
$10.00/250mg

74-88-4
341 suppliers
$15.00/10g
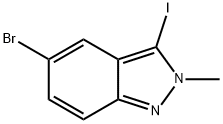
865156-35-0
14 suppliers
inquiry

865156-34-9
18 suppliers
inquiry