
5-Bromo-4-hydrazinyl-2,6-dimethylpyrimidine synthesis
- Product Name:5-Bromo-4-hydrazinyl-2,6-dimethylpyrimidine
- CAS Number:69696-36-2
- Molecular formula:C6H9BrN4
- Molecular Weight:217.07
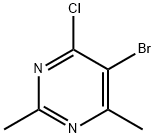
69696-35-1
38 suppliers
$69.00/100mg

69696-36-2
9 suppliers
inquiry
Yield:69696-36-2 91%
Reaction Conditions:
with hydrazine hydrate in methanol;ethanol at 0 - 20; for 16 h;
Steps:
B1.3 5-Bromo-4-hydrazinyl-2,6-dimethylpyrimidine (B1.3)
5-Bromo-4-hydrazinyl-2,6-dimethylpyrimidine (B1.3) (0349) To a mixture of Hydrazine hydrate (NH2NH2.H2O, 32 g, 0.64 mol, 98%) in 350 mL ethanol was added a solution of B1.2 (70 g, 0.32 mol) in 350 mL methanol dropwise at 0° C. The reaction mixture was stirred at rt for 16 h. The solvent was removed by reduced pressure, the residue was diluted with 500 mL of water, extracted with CHCl3 (500 mL×3). The combined organic layers were washed with 500 mL brine, dried over Na2SO4, and concentrated to give the title compound (63 g, 91%) as a yellow solid. LC-MS: [M+H]+=219.0.
References:
US2016/176882,2016,A1 Location in patent:Paragraph 0346; 0349
6622-92-0
204 suppliers
$9.00/1g

69696-36-2
9 suppliers
inquiry
858269-28-0
23 suppliers
$45.00/50mg

69696-36-2
9 suppliers
inquiry