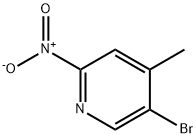
5-bromo-4-methyl-2-nitropyridine synthesis
- Product Name:5-bromo-4-methyl-2-nitropyridine
- CAS Number:1225278-70-5
- Molecular formula:C6H5BrN2O2
- Molecular Weight:217.02
98198-48-2
365 suppliers
$5.00/1g

1225278-70-5
12 suppliers
inquiry
Yield:1225278-70-5 68.95%
Reaction Conditions:
with sulfuric acid;dihydrogen peroxide at -20 - 30; for 0.75 h;Inert atmosphere;
Steps:
33.1 5-bromo-4-methyl-2-nitropyridine
Step 1
5-bromo-4-methyl-2-nitropyridine
H2O2 (58.06 g, 1.71 mol, 21.28 equivalents) was slowly added dropwise to H2SO4 (184.00 g, 1.88 mol, 23.39 equivalents) which was cooled to 0°C, and the temperature was maintained below -20°C during the dropwise addition.
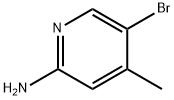
A mixture of 5-bromo-4-methylpyridine-2-amine (15.00 g, 80.20 mmol, 1.00 equivalent) in H2SO4 (100,00 mL) was then added thereto.
The reaction mixture was first stirred for 45 minutes in an ice bath and then warmed to 30°C.
After three hours, the color of the reaction solution changed gradually from grass green to bright yellow.
Ice (500 mL) was added to the reaction mixture.
The precipitated solid was filtered, washed with water and then dried in vacuo to give the title compound (12.00 g, 55.29 mmol, 68.95% yield) as a bright orange solid without further purification. LC/MS (ESI) m/z: 216.9 (M+1), 218.9 (M+3).
References:
EP3269715,2018,A1 Location in patent:Paragraph 0061; 0366; 0367