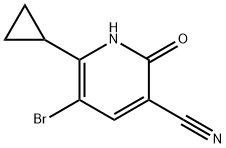
5-broMo-6-cyclopropyl-2-hydroxynicotinonitrile synthesis
- Product Name:5-broMo-6-cyclopropyl-2-hydroxynicotinonitrile
- CAS Number:1135283-57-6
- Molecular formula:C9H7BrN2O
- Molecular Weight:239.07
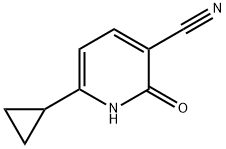
847144-72-3
15 suppliers
inquiry

1135283-57-6
8 suppliers
inquiry
Yield:-
Reaction Conditions:
with N-Bromosuccinimide in 1,2-dichloro-ethane at 20; for 3 h;Reflux;
Steps:
2.N; 1.C
Step N: 5-bromo-6-cyclopropyl-2-hydroxynicotinonitrile (14). To a solution of 6-cyclopropyl-2- hydroxynicotinonitrile (13; 0.32 g, 2.0 mmol) in 5 mL of DCE was added NBS (0.534 g, 3.0 mmol) at room temperature. The reaction mixture was heated at reflux for 3 hours. After LC-MS showed completion of reaction, the reaction mixture was cooled to room temperature and poured into water. After extraction with methylene chloride (3 x 5 mL), the combined organic layer was dried over anhy. Na2S04 and concentrated in vacuo. Column chromatography (4% MeOH/DCM) afforded 0.45 g of 14. MS (ES) M+H expected 239.0, found 238.9. 1H NMR (CHLOROFORM- d) δ 8.49-8.72 (br. s., IH), 7.93 (s,lH), 2.23 - 2.34 (m, IH), 1.36 - 1.42 (m, 2H), 1.29 - 1.36 (m, 2H).
References:
WO2012/171337,2012,A1 Location in patent:Page/Page column 113-114; 179
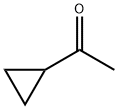
765-43-5
401 suppliers
$10.00/1g

1135283-57-6
8 suppliers
inquiry

21666-68-2
38 suppliers
$57.50/250mg

1135283-57-6
8 suppliers
inquiry