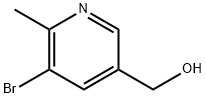
(5-broMo-6-Methylpyridin-3-yl)Methanol synthesis
- Product Name:(5-broMo-6-Methylpyridin-3-yl)Methanol
- CAS Number:1174028-23-9
- Molecular formula:C7H8BrNO
- Molecular Weight:202.0485
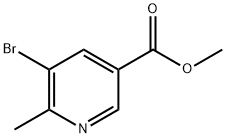
1174028-22-8
35 suppliers
inquiry

1174028-23-9
8 suppliers
inquiry
Yield:1174028-23-9 84%
Reaction Conditions:
Stage #1: methyl 5-bromo-6-methylnicotinatewith diisobutylaluminium hydride in dichloromethane;toluene at -78 - 20;Inert atmosphere;
Stage #2: with Potassium sodium tartrate in dichloromethane;water monomer;toluene;
Steps:
7.c
(c) (5-Bromo-6-methyl-3-pyridinyl)methanolTo a solution of methyl 5-bromo-6-methyl-3-pyridinecarboxylate (76 mg, 0.330 mmol) in DCM (1.5 ml), DIBAL-H (1.5 M solution in toluene, 0.661 ml, 0.991 mmol) was added dropwise at -78° C. under N2. The reaction mixture was allowed to warm to rt and stirred overnight. To this solution was added saturated, aqueous NaK-tartrate solution followed by DCM. The organic phase was separated, dried and concentrated to afford 56 mg of (5-bromo-6-methyl-3-pyridinyl)methanol (56 mg, 84%) pure enough to be used in the next step.1H-NMR (δ, ppm, CDCl3): 8.45 (s, 1H), 7.97 (s, 1H), 4.73 (s, 2H), 2.70 (s, 3H).
References:
US2009/306089,2009,A1 Location in patent:Page/Page column 29

1174028-21-7
15 suppliers
inquiry

1174028-23-9
8 suppliers
inquiry
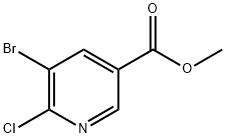
78686-77-8
214 suppliers
$6.00/1g

1174028-23-9
8 suppliers
inquiry