
5-BROMO-IMIDAZO[1,2-A]PYRIDINE HBR synthesis
- Product Name:5-BROMO-IMIDAZO[1,2-A]PYRIDINE HBR
- CAS Number:603301-13-9
- Molecular formula:C7H6Br2N2
- Molecular Weight:277.96
19798-81-3
433 suppliers
$5.00/1g
2032-35-1
479 suppliers
$5.00/10g
![5-BROMO-IMIDAZO[1,2-A]PYRIDINE HBR](/CAS/GIF/603301-13-9.gif)
603301-13-9
28 suppliers
$60.00/100mg
Yield:603301-13-9 81%
Reaction Conditions:
in butan-1-ol;Heating / reflux;
Steps:
8
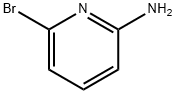
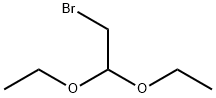
PREPARATION 8; 5-Bromoimidazo [1, 2-a] pyridine; Add 2-bromo-I, l-diethoxyethane (3.64 g, 18.48 mmol) to a solution of 6- bromopyridin-2-ylamine (l. Og, 5.77 mmol) in n-butanol (40 ml). Reflux overnight and cool. Filtration of the reaction mixture gives 1.3 g (81%) of 5-bromoimidazo [1, 2- pyridine hydrobromide as a white solid. ESMS (M++1) : 198.9 m/z. Add saturated sodium bicarbonate (300 ml) to a suspension of 5- bromoimidazo [1, 2-a] pyridine hydrobromide (13. 0g, 46.96 mmol) in ethyl acetate. Separate the organic layer, wash with saturated sodium bicarbonate, dry over magnesium sulfate, and concentrate under reduced pressure to give 9.7 g (100%) of the title compound as a white solid.
References:
WO2003/76398,2003,A2 Location in patent:Page/Page column 20