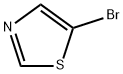
5-Bromothiazole synthesis
- Product Name:5-Bromothiazole
- CAS Number:3034-55-7
- Molecular formula:C3H2BrNS
- Molecular Weight:164.02
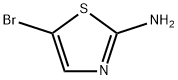
3034-22-8
210 suppliers
$7.00/250mg

3034-55-7
205 suppliers
$7.00/250mg
Yield:-
Reaction Conditions:
Stage #1:5-bromo-2-aminothiazole with phosphoric acid;nitric acid;sodium nitrite in water at -5; for 1 h;
Stage #2: with hypophosphorous acid in water at 0 - 20;
Steps:
To a solution of 2-amino-5-bromothiazole (12.58g, 70mmol) in a mixture of phosphoric acid (106ml of an 86% solution in water), and cone, nitric acid (19.2ml) cooled at - 50C was added over 45 mins a solution of sodium nitrite (7.59g, 1 lOmmol) in water (26ml). 30 After the addition was complete the mixture was stirred at -50C for 15 mins, then hypophosphorous acid (38.8ml) added dropwise over 30 mins keeping the temperature below O0C. The mixture was stirred at O0C for 150 mins then allowed to warm to room temperature overnight. The mixture was poured into a solution of NaOH (85g) in water (400ml). 5N NaOH EPO
References:
MERCK & CO., INC. WO2008/57336, 2008, A2 Location in patent:Page/Page column 40-41

4175-78-4
277 suppliers
$10.00/1 g

3034-55-7
205 suppliers
$7.00/250mg
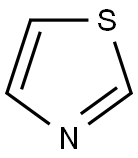
288-47-1
283 suppliers
$6.00/1g

3034-55-7
205 suppliers
$7.00/250mg
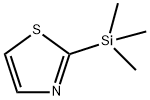
79265-30-8
185 suppliers
$22.00/1g

3034-55-7
205 suppliers
$7.00/250mg

108306-64-5
25 suppliers
inquiry