
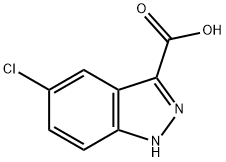
5-CHLORO-1H-INDAZOLE-3-CARBONITRILE synthesis
- Product Name:5-CHLORO-1H-INDAZOLE-3-CARBONITRILE
- CAS Number:29646-35-3
- Molecular formula:C8H4ClN3
- Molecular Weight:177.59

28751-70-4
42 suppliers
$157.03/0.5g

29646-35-3
49 suppliers
inquiry
Yield:29646-35-3 92%
Reaction Conditions:
with pyridine;trifluoroacetic anhydride in dichloromethane at 25; for 0.166667 h;
Steps:
1 5-chloro-lH-indazole-3-carbonitrile (4):
5-chloro-l H-indazole-3-carboxamide 3 (0.60 g, 3.0 mmol) was dissolved in pyridine (6 mL) and dry dichloromethane (6 mL). Trifluoroacetic acid anyhydride ( 1.0 mL, 7.7mmol) was added and the reaction stirred at 25°C for 10 minutes. The reaction mixture was concentrated in vacuo and the residue taken up in ethyl acetate, then Washed with water, saturated sodium bicarbonate and brine. The organic phase was dried over sodium sulphate, filtered and concentrated to give the title compound 5-chloro-l H-indazole-3-carbonitrile 4 (0.5 g, 92 %) as a pale yellow solid. IR Omax(film): cm" 1 2233; NMR (200 MHz, CDC13): δ 8.03 (d, J = 9.1 Hz, 1 H), 7.96 (d, J = 1 .8 Hz, 1 H), 7.75 (dd, J = 9.1 , 1 .8 Hz, 1 H).
References:
WO2015/15519,2015,A1 Location in patent:Page/Page column 18
1077-95-8
128 suppliers
$25.00/100mg

29646-35-3
49 suppliers
inquiry